Migraine is one of the most common headache disorders causing substantial disability for affected patients and has a considerable societal cost owing to the high prevalence in the general population.1 Results from the Global Burden of Diseases, Injuries, and Risk Factors Study 2015 for all-cause mortality, cause-specific mortality and non-fatal disease burden were used to calculate disability-adjusted life-years (DALYs).2 Total DALYs and all-age DALY rates for migraine significantly increased by 29.7% and 5.6%, respectively, between 1990 and 2005. Using years lived with disability (YLD), migraine is ranked seventh in the world (among the top level-4 causes of disability) for both genders and all age ranges.3 In those aged 15–49 years, migraine is ranked third. The average lifetime prevalence is approximately 18% and 1-year prevalence approximately 13%.4–10 Women experience migraine around three-times more often than men and prevalence peaks at approximately 45 years of age.11
A number of guidelines have been published and recommend the use of pharmacological therapies as first-line treatment for mild-to-moderate migraine in adults. The National Institute for Health and Care Excellence (NICE) published guidelines for England and Wales in 2015.12 These guidelines based on scientific evidence are intended for the primary care setting. At first consultation, the doctor should obtain a complete description and make the diagnosis while excluding serious underlying causes. A triptan should be prescribed plus either a non-steroidal anti-inflammatory drug (NSAID) or paracetamol to treat the attack. Topiramate, propanolol or amitriptyline can be used for prevention, and frovatriptan or zolmitriptan in menstrual migraine. Silberstein et al. assessed pharmacological therapies for the prevention of migraine and identified 29 Class I or Class II articles from published studies.13 They concluded that antiepileptic drugs (divalproex sodium, sodium valproate and topiramate), β-blockers (metoprolol, propanolol and timolol) and triptans (frovatriptan for short-term menstrual migraine prevention) were effective for migraine prevention (Level A – medicines with established efficacy in at least two Class I trials). These drugs should be prescribed to patients with migraine to reduce attack frequency and severity.13
Triptans are effective for migraine relief and their use represented a breakthrough in the treatment of migraine.14 However, it is unclear which triptan has the greatest efficacy compared with the others in this class and when compared with non-triptan migraine treatments. Cameron et al. assessed 133 randomised, controlled trials (RCTs) of triptans versus placebo or active controls. Standard doses of triptans relieved headaches within 2 hours and gave pain relief for 2 hours (42–76% and 18–50%, respectively). Furthermore, they gave sustained headache relief and freedom from pain. Generally, triptans provided equal or better outcomes than other migraine treatments but slightly worse efficacy than combination therapy.14
This review examines the use of the currently licensed triptans for the treatment of acute migraine and practical considerations when selecting a triptan for this indication. The efficacy and safety of the triptans using data from clinical trials and real-world studies will be compared.
Triptans for the treatment of acute migraine
Triptans are serotonin (5-hydroxytryptamine, or 5-HT) agonists with high affinity for 5-HT1B and 5-HT1D receptors.15 When the mode of action of triptans was first investigated, it was thought that they gave relief from migraine from their ability to cause cranial vasoconstriction, probably through action at postsynaptic 5-HT1B receptors on the smooth-muscle cells of blood vessels. More recently, it has been discovered that triptans also block the release of vasoactive peptides from the perivascular trigeminal neurons through their action at presynaptic 5-HT1D receptors on the nerve terminals.16 They also bind to presynaptic 5-HT1D receptors in the dorsal horn, which seems to block the release of neurotransmitters that activate second-order neurons ascending to the thalamus.16 In addition, they may also facilitate descending pain inhibitory systems.16 However, it is not certain whether the activation of vascular 5-HT1B receptors is essential for relieving migraine.15
There are currently seven molecules in the triptan class – almotriptan, eletriptan, frovatriptan, naratriptan, rizatriptan, sumatriptan and zolmitriptan.17 They are biochemically similar but have distinctive pharmacokinetic and pharmacodynamic properties. For example, naratriptan and frovatriptan have a longer half-life and therefore a delayed onset of action and prolonged duration compared with the other triptans, which are fast acting, with a rapid dose-dependent efficacy and higher risk of adverse events (AEs) and recurrence of the migraine. Thus, many drug characteristics need to be considered when selecting the optimum triptan for an individual patient.
Almotriptan
Almotriptan is a potent, selective 5-HT1B and 5-HT1D receptor agonist with approximately 70% bioavailability when administered orally, which is higher than that for other triptans.18 Although the mean half-life is similar to other triptans (3–5 hours), it is significantly lower than frovatriptan (half-life approximately 25 hours).18 Almotriptan has been shown to be one of the best-responding triptans for pain relief and pain-free rate at 2 hours with the lowest AEs of the triptans.18
The activity of almotriptan has been evaluated in four controlled, double-blind, randomised clinical trials. Two were against placebo and two included sumatriptan (n=2,500 patients with moderate-to-severe episodic migraine).18 The first trial assessed almotriptan at 6.25 or 12.5 mg.19 Pain relief at 2 hours was significantly higher with almotriptan (60, 70 and 38% for the 6.25, 12.5 mg dose of almotriptan and placebo respectively, p<0.001). AEs occurred in 23.1% of patients, but there were no significant differences between groups. A dose-finding phase II trial assessed a single dose of almotriptan (2, 6.25, 12.5 and 25 mg) versus placebo in 742 patients.20 The headache response at 2 hours showed a significant dose-dependent increase (p<0.0001), with efficacy being greater than placebo (p<0.001) except for the 2 mg dose. In total, 18.7% of patients had AEs with the incidence and intensity dose-dependent and they were significantly higher at the 25 mg dose. In contrast AEs were similar to placebo for the other doses. These findings indicated that the optimum dose for almotriptan was 12.5 mg.
Almotriptan (12.5, 25 mg) was compared to sumatriptan (100 mg) in a randomised, single-dose, double-blind, parallel-group, placebo-controlled, multicentre trial.21 One moderate or severe migraine attack was treated in 668 patients.18 All treatments significantly improved response rates compared with placebo (56.8, 56.5, 63.7 and 42.4% for almotriptan 12.5, 25 mg, sumatriptan 100 mg and placebo, respectively). Sumatriptan gave superior results for pain relief at 2 hours stratified by intensity of attack, but a higher percentage of patients were pain-free at 1 and 2 hours with either almotriptan dose. The rate of AEs was very low with all doses.
A further trial compared almotriptan (12.5 mg) with sumatriptan (50 mg) in 1,173 patients with moderate or severe migraine.22 The findings showed that both treatments gave a similar rate of pain relief at 2 hours, but sumatriptan provided significantly better pain-free status (p=0.05).18 Migraine-associated symptoms and recurrence rates within 24 hours were similar with both treatments. No serious AEs were reported in any patient.
The sustained pain-free rate has been evaluated in a pooled analysis of three randomised, double-blind, placebo-controlled trials with almotriptan versus placebo or sumatriptan.23 Almotriptan treatment(6.25 and 12.5 mg) resulted in significantly better-sustained pain-free rates (p<0.05 versus placebo). Similar numbers of patients achieved sustained pain-free status in those treated with almotriptan or sumatriptan.23
Almotriptan is an effective and well-tolerated treatment for acute migraine and has been compared with rizatriptan in an open-label, crossover study in a real-world setting.24 A sub-study from a multicentre, open-label, crossover trial was conducted in 146 patients for the acute treatment of two migraine attacks using a sequential, crossover protocol.24 Significantly more patients taking rizatriptan had pain relief onset and more achieved pain freedom within 2 hours of dosing compared with those taking almotriptan (88.6 versus 73.4%, p=0.007 and 55.7 versus 45.6%, each p=0.1, respectively). In addition, in the rizatriptan group times to onset of pain freedom and pain relief were shorter than in the almotriptan group (median time 45 versus 60 minutes, p=0.002 and 100 versus 135 minutes, p=0.004 for pain relief and pain freedom, respectively).24 Of note, most patients preferred rizatriptan.24
Eletriptan
Eletriptan exhibits high affinity, selectivity and potent agonistic activity to human 5-HT1B and 5-HT1D receptors.25 Affinity to the human 5-HT1B and 5-HT1D receptors is higher than observed with sumatriptan for the cranial artery compared with the coronary artery.25 Eletriptan is also rapidly absorbed and has good bioavailability following oral administration.25 Results from clinical trials indicate that eletriptan rapidly improves headache response and reduces headache recurrence. In addition, functional impairments are improved and the majority of AEs are mild-to-moderate.25
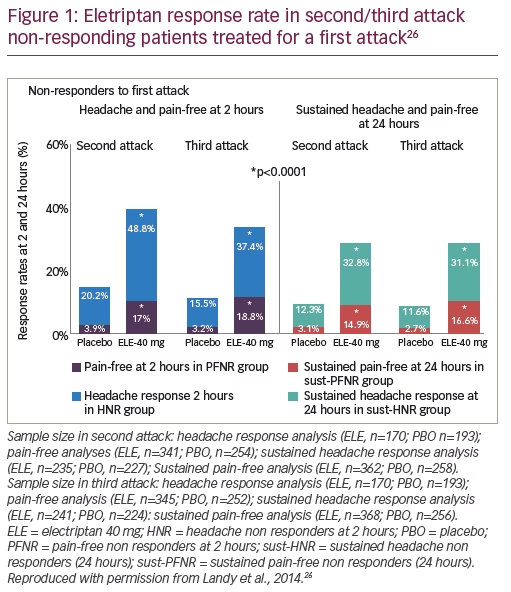
Eletriptan (40 mg) has been shown to be one of the most effective acute migraine treatments in several meta-analyses.26 Furthermore, a post hoc analysis showed that patients who did not respond to the 40 mg dose for three consecutive migraine attacks had a positive response to eletriptan 80 mg versus placebo at 2 hours with the next three attacks (p<0.05 for all comparisons); dose escalation may represent an effective strategy for around 50% of patients who show no response at a lower dose.26
Another analysis using results pooled from four randomised, double-blind, placebo-controlled trials of eletriptan treatment for multi-attack migraine evaluated the effect of treating a second and third attack with the same 40 mg dose in those who did not respond in the first attack to this treatment.26 In total 1,299 patients were combined from the studies with 297 who received eletriptan 40 mg and 375 placebo, having no response to the first attack at 2 hours. Headache response at 2 hours for the first treated attack was significantly higher for treated patients compared with placebo (65.5 versus 28.7%, p<0.0001). Similar favourable results occurred with respect to pain-free at 2 hours, sustained headache response at24 hours and sustained pain-free at 24 hours for eletriptan versus placebo (all p<0.0001). In non-responding patients to the first attack, eletriptan treatment was significantly superior to placebo for second and third attacks on all efficacy measures (all p<0.0001) (Figure 1). These findings suggest that patients who do not respond to an initial dose of eletriptan may respond with the same dose to a second or third attack.26
Although eletriptan (80 mg) has shown significantly superior efficacy to sumatriptan (50 or 100 mg) in two clinical trials, this was only seen at a dose of 40 mg in one trial.27 Therefore, eletriptan (40 mg) has been compared with sumatriptan (100 mg) in a randomised, double-blind, double-dummy, parallel-group trial (n=2113) in single migraine attack to confirm the previous results.27 Eletriptan gave a significantly higher response rate at 2 hours than sumatriptan (67 versus 59%, respectively, p<0.0001). For other endpoints, including 1-hour headache response, 2-hour pain-free response, rescue medication use and sustained headache response, eletriptan was also superior (p<0.05). Treatment-related AEs were low with nausea the only one with a ≥2% incidence (eletriptan 4.9%, sumatriptan 4.2% and placebo 2.8%). This study therefore confirmed the better efficacy and tolerability with eletriptan than sumatriptan for treating migraine pain and restoring patient functioning.27
Frovatriptan
Frovatriptan has an extremely long half-life of approximately 25 hours, which is around five-times that of other triptans.28 Frovatriptan has high affinity for 5-HT1B and 5-HT1D receptors and moderate affinity for 5-HT1A and 5-HT1F. It is one of the most potent 5-HT1B agonists.28
Frovatriptan has distinctive pharmacokinetic and pharmacological properties, and was developed to offer a long duration of action and low likelihood of drug interactions and side effects.29–31 It is more selective for cerebral arteries compared with coronary arteries and is thus more suitable for treating those at risk from coronary artery disease. Although frovatriptan is mainly metabolised by cytochrome P450 (CYP) 1A2 it does not affect (inhibit or induce) this or other CYP isoenzymes. It also is not a substrate for monoamine oxidase-A in contrast to some other triptans and does not bind to plasma proteins to a large extent, hence it has a low risk of pharmacokinetic drug interactions.31
Results from three randomised, placebo-controlled, double-blind, parallel-group trials (2,676 migraine patients) indicated that headache response after 2 hours was significantly greater with frovatriptan compared with placebo (p≤0.001) and was also approximately twice as effective at 4 hours.32 Furthermore, in most patients, time to headache response was within 1.5 hours and there was a low incidence (10–25%) of 24-hour headache recurrence.
Frovatriptan has been evaluated in three double-blind, cross-over, head-to-head trials versus rizatriptan,33 zolmitriptan34 and almotriptan.35
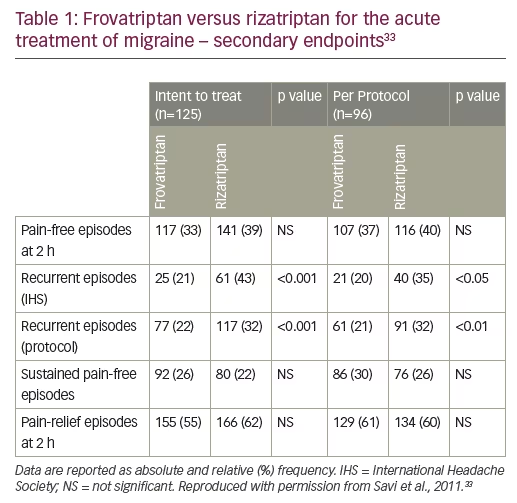
The main aim of the study comparing frovatriptan and rizatriptan was to evaluate patient satisfaction for the treatment of acute migraine with either drug.33 Patients were recruited with a history of migraine and one or more attack in the previous 6 months. Treatment of 1–3 attacks for <3 months with either frovatriptan (2.5 mg) or rizatriptan (10 mg) was compared. The mean preference scores for 104 patients were similar for both treatments (2.9±1.3 versus 3.2±1.1 for frovatriptan and rizatriptan, respectively). Furthermore, there was no significant difference for the secondary endpoints of rates of pain-free and pain-relief episodes at 2 hours or sustained pain-free episodes. However, frovatriptan resulted in significantly lower recurrent episodes than rizatriptan (21 versus 43% respectively, p<0.001) (Table 1). The number of patients with AEs was also similar. Thus, the efficacy of these two triptans is similar but frovatriptan has a greater duration of action.33
Frovatriptan (2.5 mg) and zolmitriptan (2.5 mg) were compared in a study of 133 patients with a history of migraine using similar endpoints to the trial reported by Savi et al.34 Both treatments were assigned similar preference scores (2.9±1.3 versus 3.0±1.3 for frovatriptan and rizatriptan, respectively). No significant differences were noted for pain-free or pain-relief episodes at 2 hours, recurrence rate, or sustained pain-free, but recurrence time favoured frovatriptan in particular between hours 4–16 (p<0.05). Significantly fewer treatment-related AEs occurred with frovatriptan than zolmitriptan (p<0.05). In this study, frovatriptan offered the advantage of the two triptans with respect to recurrence and tolerability.34
The third study comparing frovatriptan (2.5 mg) and almotriptan (12.5 mg) had a very similar design with respect to patients enrolled and endpoints.35 In total, 114 patients completed the preference questionnaire. As with the other head-to-head studies there was no significant difference in patient preference, rates of pain-free (2 or 4 hours) and pain-relief (2 hours), sustained pain-free episodes and recurrent episodes were significantly less frequent with frovatriptan (30 versus 44% with almotriptan, p<0.05) and for recurrent episodes treated within 30 minutes (p<0.05). Tolerability was also similar.35
The results from each of these three studies indicated that the triptans had similar efficacy in the immediate treatment of migraine, although frovatriptan was associated with lower recurrence rates, and thus improved sustained relief. However, in these studies, patients were instructed to take the drug as soon as the headache appeared (according to product labelling of all triptans).36–42 This could explain why no difference was seen between frovatriptan and other triptans at 2 hours. Moreover, the definition of recurrence used in these studies was from the International Headache Society Guidelines43 that is the more severe and affordable, and differs from many recurrence definitions used in a number of clinical trials of triptans.
Naratriptan
The efficacy of naratriptan (2.5 mg) treatment for 1 year was assessed in an open-label study in acute migraine attacks (n=417).44 Treated patients had headache relief in a median of 70% of moderate or severe and 86% of mild attacks, 4 hours after dosing. Furthermore, headache relief remained the same over prolonged treatment (0–6 versus 6–12 months) and frequency of naratriptan use (up to or over 36 attacks). AEs were absent in 84% of treated attacks and did not increase with respect to the number of doses.44
A comparative randomised, double-blind, crossover study comparing naratriptan with sumatriptan in 253 migraine patients (safety analysis) with a history of frequent headache recurrence has been reported.45 The efficacy analysis included 225 patients who treated two attacks. Headache recurrence occurred in 45% naratriptan-treated patients and 57% treated with sumatriptan 4–24 hours after treatment (not statistically significant). However, in patients with headache relief after two attacks 4–24 hours post-dosing, recurrence was more likely in the sumatriptan group (41 versus 57% respectively, p=0.005). AEs were recorded in 22% of patients (naratriptan group) and 33% for sumatriptan, and the incidence was similar following a second dose. In this study, these triptans gave similar efficacy, although in those who had headache relief after two attacks recurrence was significantly lower with naratriptan.45
A meta-analysis of 10 RCTs (4,499 patients) with moderate or severe migraine attacks indicated that the rate ratio (RR) versus placebo for naratriptan 2.5 mg was 2.52 (95% confidence interval [CI] 1.78–3.57) and 2.58 (1.99–3.35) for pain-free response at 2 and 4 hours.46 However, it was less effective in pain-free response than either rizatriptan or sumatriptan. In contrast, AEs were significantly fewer with naratriptan.46
Naratriptan has been evaluated as a preventative treatment of refractory chronic migraine compared with other treatments.47 In total, 27 patients who had failed on at least four preventative drugs were prescribed naratriptan twice daily (2.5 mg). The frequency of headache days was significantly reduced by naratriptan at 2, 6 and 12 months (p<0.001) and the number of days/month of severe pain significantly reduced at 1, 2, 6 and 12 months (p <0.01 for all time points). The headache index was also reduced at 2, 6 and 12 months (all p<0.001).47
Rizatriptan
Rizatriptan is rapidly absorbed, achieving plasma concentrations more quickly than other triptans.7 As with other triptans, rizatriptan causes contraction of isolated human coronary arteries in vitro – the effect occurs at high concentrations so myocardial ischaemia is unlikely to occur at plasma concentrations in patients with normal coronary circulation. It is available either as a tablet or as a wafer (orally disintegrating tablet [ODT]).48
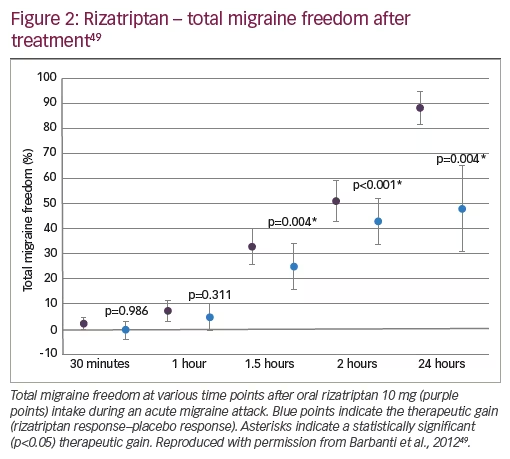
The efficacy of rizatriptan has been demonstrated in randomised, double-blind, placebo-controlled trials. In one study, 80 patients with unilateral cranial autonomic symptoms were treated for a single moderate or severe migraine attack with 10 mg rizatriptan (wafer) or placebo.49 Freedom from pain 2 hours after treatment occurred in 54 and 8% with rizatriptan versus placebo, respectively (46% therapeutic gain, p<0.001). In addition, significantly more rizatriptan-treated patients had total migraine freedom at 2 hours compared with placebo (51 and 8% respectively; therapeutic gain 43%, p<0.001) (Figure 2).49
Ferrari et al. conducted a meta-analysis of seven randomised, placebo-controlled, double-blind phase III trials of rizatriptan (10 mg, n=2,068 or 5 mg, n=1,486) versus placebo (n=1,260) for treating an acute migraine attack.50 Rizatriptan (10 mg) was significantly better compared with placebo for pain relief at 2 hours (71 versus 38%, p<0.001) and for the elimination of pain, nausea, photophobia, phonophobia and functional disability. In addition, more patients treated with rizatriptan than placebo had sustained pain relief over 24 hours (37 versus 18% respectively, p<0.001). Except for the elimination of nausea, the 10 mg dose was significantly more effective than 5 mg on all endpoints at 2 hours and maintained over 24 hours (38 versus 32% for rizatriptan 10 mg and 5 mg, respectively, p=0.001).50
Rizatriptan (5 mg for body weight 20–39 kg, 10 mg >40 kg) has also been evaluated in 96 young patients aged 6–17 years in a double-blind, placebo-controlled, cross-over trial.51 Patients receiving either dose of rizatriptan achieved headache relief by two grades at 2 hours compared with placebo (p<0.001). Rizatriptan was superior at 3 and 4 hours and was consistent over two treated attacks. Serious AEs were not evident.51
Sumatriptan
Sumatriptan is considered the gold standard triptan and is administered orally, subcutaneously (SC) or as a nasal spray. A Cochrane review considered the efficacy and tolerability of oral sumatriptan for treating a single acute migraine attack.52 Double-blind, RCTs were identified in which oral sumatriptan (100, 50 and 25 mg) was evaluated against placebo, no intervention, other drug treatments, behavioural therapy or physical therapy for an acute migraine attack in adults. In total, 25 RCTs with 16,200 patients were included. In the placebo-controlled trials, all doses of sumatriptan gave headache relief and relief of disability at 2 hours. With respect to pain-free response at 2 hours, numbers-needed-to-treat (NNT) were 5.1 (3.9–7.1), and 7.5 (2.7–142) for the 100 mg and 25 mg dose, respectively. However, there was no significant difference between placebo and 50 mg. The NNT were similar with the three doses for headache relief. Sumatriptan was well tolerated, but AEs were more common with the 100-mg dose compared with placebo (risk difference [RD] 0.14). There were no significant differences for RDs between the 50 and 25 mg doses and placebo. The authors concluded that oral sumatriptan is well tolerated and effective for treating a single acute migraine attack. Other triptans had similar effectiveness, but ergotamine plus caffeine was significantly less effective than sumatriptan.52
Many peripheral and central neural mechanisms contribute to the pathogenesis of migraine and have been identified as targets for acute and preventative treatment. Accordingly, treatment of migraine with a triptan and NSAID class, which have different mechanisms of action, can give better relief of migraine.53 This strategy was evaluated in a multicentre, randomised, double-blind, double-dummy, placebo-controlled, four-arm study in 972 patients. A single moderate or severe migraine attack was treated with either naproxen sodium (500 mg), sumatriptan (50 mg), a combination of sumatriptan and naproxen (50 and 500 mg, respectively) or placebo. Sumatriptan plus naproxen was significantly more effective than sumatriptan or naproxen alone in achieving pain relief for 24 hours (46, 29 and 25 versus 17% for the combination, sumatriptan alone, naproxen alone or the placebo group, respectively [p<0.001]). In addition, sumatriptan plus naproxen was significantly better than monotherapy in the 2-hour headache response, 2-hour pain-free and sustained pain-free responses (p<0.001) AEs were similar in all treatment groups.53
A total of 12 randomised, double-blind, placebo- or active-controlled studies of sumatriptan plus naproxen to treat an episode of migraine headache have been reviewed.54 Patients (n=3,663) were treated for mild, moderate or severe pain attacks with sumatriptan (85 or 50 mg) plus naproxen (500 mg). The findings showed that the combination was superior to placebo for pain-free and headache relief at 2 hours. The higher dose of sumatriptan did not significantly alter the findings, but early treatments were significantly more effective. Although AEs were generally mild or moderate, they were more common with the combination compared with placebo. Thus, the combination treatment was more effective than the same dose of either drug alone.54 Sumatriptan plus naproxen is now available in combination form.55
Zolmitriptan
An open-label phase II study of zolmitriptan permitted patients to choose treatment for initial, persistent or recurrent migraine headache.56 In total, 49,784 patients were treated with 2.5 or 5 mg zolmitriptan and 66% used a single dose. The 2-hour headache and pain-free response rate for all attacks was 85 and 79%, and 69 and 59%, respectively, for an initial dose of 2.5 or 5 mg zolmitriptan. In addition, both doses of zolmitriptan gave good headache (84–91% and 76–84%, respectively) and pain-free response rates (70–76% and 58–65%, respectively) in patients treating at least 20 attacks. Furthermore, gender, age, presence of aura or relationship to menses had no effect on the treatment of multiple migraine attacks.56
Zolmitriptan has been assessed in a multicentre, randomised, double-blind, placebo-controlled study in 2,122 migraine patients with or without aura.57 Zolmitriptan was administered as a 5 mg nasal spray for the treatment of up to two migraine attacks within 15 minutes of pain being moderate or severe. Zolmitriptan treatment gave a significant headache response at 2 hours and at all earlier time points as early as 15 minutes post-dose (66.2% of treated patients compared with 35% on placebo, p<0.001 for headache response at 2 hours and p<0.001 for earlier time points). Zolmitriptan treatment was also better than placebo based on pain-free rates, and headache response at 4 hours, and sustained headache response and pain-free response at 24 hours. AEs were mild or moderate and of short duration with zolmitriptan. These results indicate that zolmitriptan is highly effective in treating acute migraine with very fast onset of action (15 minutes post-dose), a sustained response and good tolerability. 57
Zolmitriptan has also demonstrated efficacy and good tolerability for the treatment of menstrual migraine attacks in a multicentre, randomised, double-blind, placebo-controlled, parallel-group outpatient study.58
The Zomig treatment of acute migraine headache in adolescents (TEENZ) study assessed the efficacy of zolmitriptan nasal spray versus placebo for the acute treatment of a single episode of adolescent migraine in patients 12–17 years of age.59 In this randomised, double-blind, placebo-controlled trial 789 patients were treated with either 5, 2.5, or 0.5 mg zolmitriptan nasal spray or placebo. An interim analysis showed that doses of 0.5 and 2.5 mg were ineffective compared with placebo, but zolmitriptan 5.0 mg was significantly more effective than placebo (p<0.001). Zolmitriptan 5.0 mg was also significantly better than placebo with respect to pain-free status 3 or 4 hours post-treatment and in achieving headache response after 2, 3 and 4 hours (p<0.001 and p≤0.011). All doses of zomitriptan were well tolerated with dysgeusia the most frequent AE and no serious AEs or AEs leading to discontinuation. Furthermore, the majority of AEs were mild or moderate and consistent with the known profile of zomitriptan in adult and adolescents.59
Serotonin syndrome
When triptans and selective serotonin reuptake inhibitors (SSRIs) or serotonin-noradrenaline reuptake inhibitors (SNRIs) are prescribed together, there is a risk of potentially fatal serotonin syndrome.60 A warning is triggered with most drug interaction programmes when these drugs are co-prescribed. In 2006 the US Food and Drug Administration issued a warning for this risk, but there is a lack of consensus as the number of cases of serotonin syndrome are extremely low, the interaction is biologically implausible and triptans are used relatively infrequently. The American Headache Society concluded that there are inadequate data to assess the risk and evidence does not support limiting the use of the combination, but caution is needed when a triptan and SSRI or SNRI are administered.61 Generally, the AEs associated with triptans occur infrequently and are benign. Central nervous system AEs have been reported in up to 15% of patients, but vary among triptans with almotriptan, naratriptan and sumatriptan having the lowest incidence.62
Triptans are contraindicated with a number of conditions including cardiovascular disease, during pregnancy and breastfeeding, in combination with monoamine oxidase inhibitors or ergot compounds, and for familial hemiplegic migraine and basilar migraine.36–41,63–65
Practical considerations when selecting a triptan for the treatment of migraine
Triptans are the first-line option to immediately treat acute moderate-to-severe migraines. Each triptan has different pharmacokinetic and pharmacodynamic properties and formulation. Some have several formulations such as tablets, dissolvable tablets, nasal sprays and injections. Many studies have been completed with triptans but they have not all been tested head-to-head in a single study. In order to select the optimum triptan for an individual patient, efficacy, safety and both pharmacokinetic and pharmacodynamic properties should be considered. In addition, the clinical characteristics of the migraine attack and the lifestyle of the patient and medical history are important.64
The properties of triptans can guide the optimum drug to prescribe for different migraine attacks. For example, where there is a high rate of recurrence, a long-, fast-acting triptan is most suitable to achieve rapid peak intensity or nocturnal onset, and triptans administered by SC injection, nasal spray, or suppository for early onset, or where there is a preponderance of vegetative symptoms.64
Meta-analyses of triptan treatment have been published. Cameron and colleagues conducted a systemic review and network meta-analysis comparing the relative efficacy of triptans using the Cochrane Library, Medline and Embase (133 randomised trials).14 The main efficacy variables (headache relief within 2 hours, 2-hour sustained freedom from pain, sustained headache relief and freedom from pain at 24 hours) were met by standard doses of triptans with rates from 18–76% of patients. Sumatriptan (SC), rizatriptan (ODT), zolmitriptan (ODT) and eletriptan tablets were associated with the best outcomes.14
A similar network meta-analysis comparing NSAIDs and triptans concluded that eletriptan was probably the most suitable drug, although the excellent tolerability of ibuprofen makes that a good choice too. Combination therapy may also be a promising future approach.66
Thorlund et al. conducted a Bayesian multiple treatment comparison meta-analysis of seven triptans used in adults to abort migraine attacks from 74 double-blind, randomised clinical trials.67 Although all triptans were significantly superior to placebo (odds ratio [OR], 95% credible interval precluding 1.00), eletriptan produced the most favourable outcome in producing sustained pain-free responses.67
All triptans are effective compared with placebo at marketed doses with good tolerability. Treatment-related AEs are generally mild and transient i.e., quickly disappear.
Conclusions
All of the currently licensed triptans are effective in a number of manifestations of migraine. They have a number of different formulations which have to be considered when a triptan is prescribed and it is important that the most effective one is selected for each patient. Studies have shown that the formulations do not have the same efficacy in every indication, for example zolmitriptan nasal spray. In addition, published meta-analyses of studies have provided information to assist prescribers to choose the best option. Generally, AEs are mild to moderate with all the triptans. However, there is currently no consensus as to the role of triptans with respect to serotonin syndrome, but doctors need to be aware of the possible problems when triptans and SSRIs or SNRIs are co-prescribed. Triptans are contraindicated with several diseases especially those of the cardiovascular system.
The third edition of the International Headache Society guidelines for controlled trials of drugs in migraine includes recommendations for the incidence of a recurrence.68 After 2-hour pain freedom, any headache pain from 2–48 hours following study drug administration, regardless of its severity, should be considered a recurrence. Previously recurrence or relapse has been defined as occurring when a study participant gains pain relief initially (improvement for moderate or severe pain at baseline to mild or no pain at the primary efficacy time point) and subsequently experiences a moderate or severe headache from the time point of primary efficacy and up to 24 hours. The main drawback associated with triptans is headache recurrence and the underlying mechanisms are not well understood.69 Rates of recurrence vary with each triptan. Geraud et al. investigated mean recurrence rates from the results of 31 triptan efficacy studies.69 Frovatriptan gave the lowest rate with rizatriptan the highest (17 and 40%, respectively), with elimination half-life inversely correlated with recurrence (p=0.0016). Furthermore, 5-HT1B but not 5-HT1D receptor potency was significantly correlated with recurrence (p=0.034 and 0.54, respectively). Migraine recurrence is affected by the pharmacological and pharmacokinetic properties of the triptan but not related to initial clinical efficacy. The triptans with a longer half-life and largest 5-HT1B receptor affinity have the lowest rates of headache recurrence.69
Triptans are effective treatments for acute migraine, but have different properties in terms of efficacy, tolerability and pharmacokinetics and pharmacodynamics. These attributes need to be taken into consideration when selecting the optimum triptan for treating a patient with acute migraine. Furthermore, combination therapy with a triptan and another drug should be explored as this approach may enhance their effectiveness.







