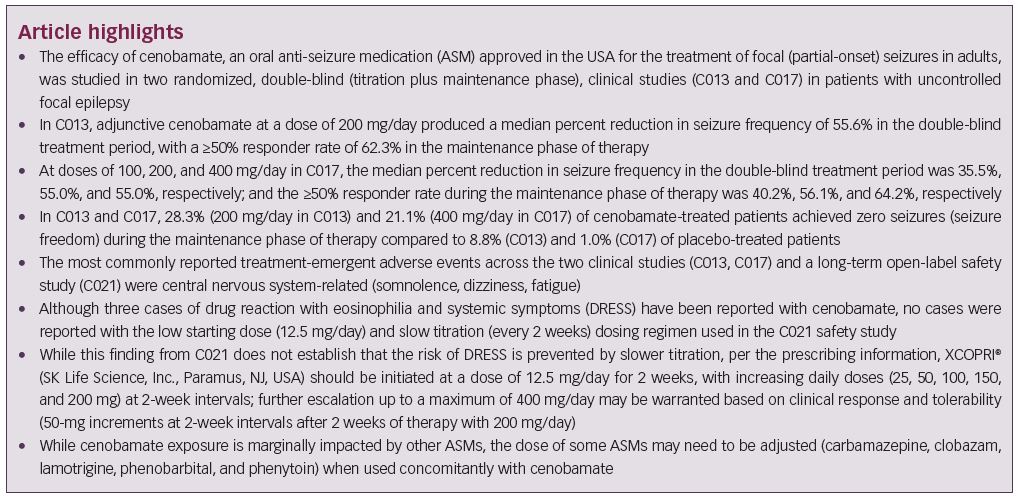
Epilepsy is a very common neurological disease, affecting more than 50 million people worldwide and 3.4 million people in the USA.1–3 Focal seizures, formerly partial-onset seizures, are the most common type, making up ≥60% of cases.4–6 Patients with epilepsy have an increased risk of morbidity and mortality, a decreased quality of life and are more likely to have cognitive deficits, psychiatric disorders, and emotional and psychosocial difficulties.2,7 Pharmacotherapy with an anti-seizure medication (ASM)/antiepileptic drug, used as monotherapy or in combination, is the predominant treatment modality;8,9 however, adverse effects and drug interactions may contribute to treatment failure.10,11 Over 35% of patients continue to have seizures despite the use of various concomitant ASMs, and the likelihood of achieving seizure freedom decreases with each subsequent ASM regimen.12,13 Patients with uncontrolled seizures may develop comorbidities such as memory and cognitive impairment, psychiatric disease, reproductive endocrine disease, and structural changes to the brain, and are at greater risk for sudden unexpected death in epilepsy and premature death.7,14,15 This review focuses on cenobamate, a newly available ASM, and the clinical data regarding its efficacy and safety in uncontrolled focal (partial-onset) seizures.
Cenobamate pharmacodynamics and pharmacokinetics
Cenobamate is an oral ASM approved in the USA for the treatment of focal (partial-onset) seizures in adults.16,17 The precise mechanism by which cenobamate exerts its therapeutic effects in patients with focal seizures in unknown.17 Cenobamate has been demonstrated to reduce repetitive neuronal firing by inhibiting voltage-gated sodium currents, with preferential inhibition of the persistent current.17,18 It is also a positive allosteric modulator of the γ-aminobutyric acid (GABAA) ion channel,17,19 binding to non-benzodiazepine GABAA receptor binding sites.19
Following oral administration, 88% of cenobamate is absorbed.17 No significant alterations in pharmacokinetic (PK) parameters were seen following a high-fat meal (800–1,000 calories with 50% fat).17 Cenobamate may be taken with or without food at any time and should be swallowed whole with liquid. PK data demonstrated dose-proportional increases in Cmax and the area under the concentration-time curve (AUC) after multiple dosing.17,20 The volume of distribution is 40–50 L with 60% plasma protein binding, the majority of which is to albumin.17 Cenobamate has a terminal half-life of 50–60 hours, which allows for once-daily dosing, with steady state reached at approximately 2 weeks.17,20 Cenobamate is metabolized via glucuronidation, oxidation, and hydrolysis in the liver to inactive metabolites.17,20,21 The only metabolite in the plasma accounts for 1.2% (standard deviation 0.67) of the parent drug.21 Cenobamate and its metabolites are primarily excreted in the urine (approximately 88% of the administered dose).17,21
After a single dose of cenobamate 200 mg, the AUC was 1.4- and 1.5-times higher in subjects with mild (creatinine clearance [ClCr] 60–<90 mL/min) and moderate (ClCr 30–<60 mL/min) renal impairment. Cenobamate AUC was 2.1- and 2.3-times higher in patients with mild (Child–Pugh Class A, 5–6 points) and moderate (Child–Pugh Class B, 7–9 points) hepatic impairment after a single dose of cenobamate 200 mg. Given the metabolism of cenobamate in the liver and the excretion of cenobamate and its metabolites in the urine, caution and dose reductions may be needed in patients with renal and/or hepatic impairment.17 A lower dose should be considered in patients with ClCr <90 mL/min and the maximum recommended dose with Child–Pugh Class A or B hepatic impairment is reduced by half to 200 mg once daily. PK data are not available in patients on hemodialysis or patients with severe hepatic impairment. No clinically relevant differences in PK were seen based on age in adults ranging from 18–77 years old. Renal and hepatic impairment, cardiac or other concomitant diseases and concurrent drug therapy should be taken into account when selecting the dose for elderly patients, starting with doses in the lower end of the dosing range.17
Preclinical and early studies
Rodent models demonstrated that cenobamate protects against maximal electroshock-induced seizures and pentylenetetrazol/picrotoxin-induced clonic seizures.22 It exhibited activity in lithium/pilocarpine-induced status epilepticus, hippocampal kindled and psychomotor seizures, and in spike-and-wave discharges in the genetic absence epilepsy rat from Strasbourg model.22,23 Altogether, preclinical studies confirmed that cenobamate has broad-spectrum activity with effects in focal, generalized, and absence seizures. Central nervous system (CNS)-related adverse events, such as somnolence, dizziness and gait disturbances, and nausea were reported in phase I studies.22,24 A proof-of-principle phase IIa study in patients with photosensitive epilepsy established that partial suppression of intermittent photic stimulation-induced photoparoxysmal response occurred with a 100 mg cenobamate dose.25
Cenobamate efficacy
Two clinical studies have evaluated the efficacy of adjunctive cenobamate for the treatment of uncontrolled focal (partial-onset) seizures in adults, the details of which are listed in Table 1. In the first study, C013 (ClinicalTrials.gov identifier: NCT01397968), adults with uncontrolled focal seizures, despite ongoing treatment with one to three ASMs, were randomized to cenobamate or placebo for 12 weeks, including 6-week titration and 6-week maintenance phases. A total of 222 patients were treated. The five most frequent concomitant ASMs in the cenobamate group were levetiracetam, lamotrigine, carbamazepine, lacosamide, and topiramate. Cenobamate was initiated at 50 mg once daily, a higher starting dose than is currently recommended in the XCOPRI® (SK Life Science, Inc., Paramus, NJ, USA) prescribing information, and titrated every other week by 50-mg increments to a target maintenance dose of 200 mg/day.17,26
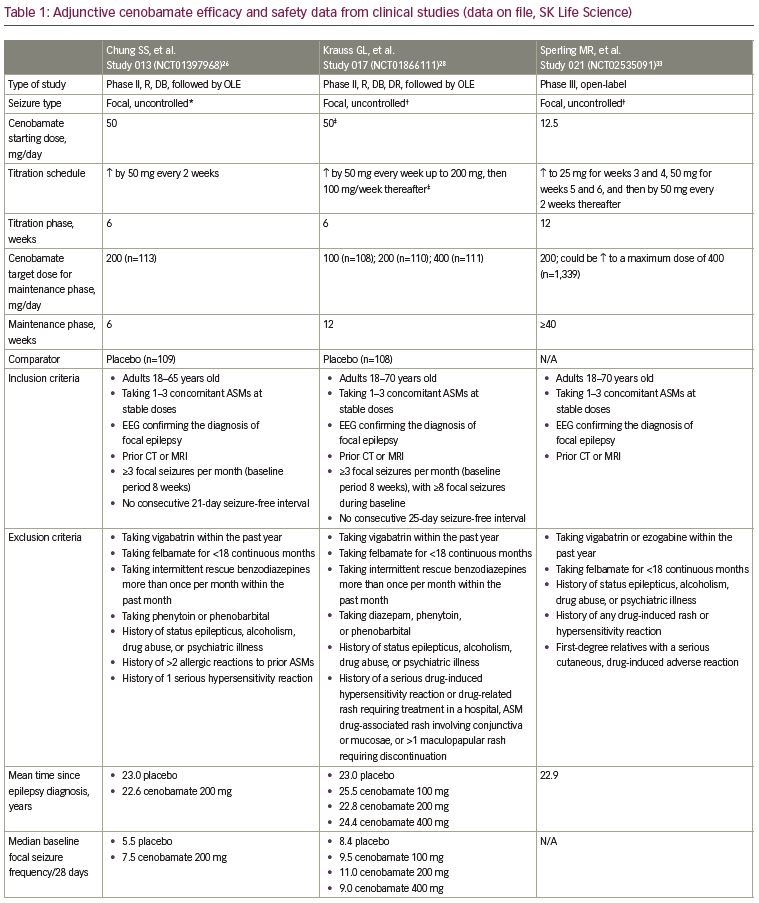
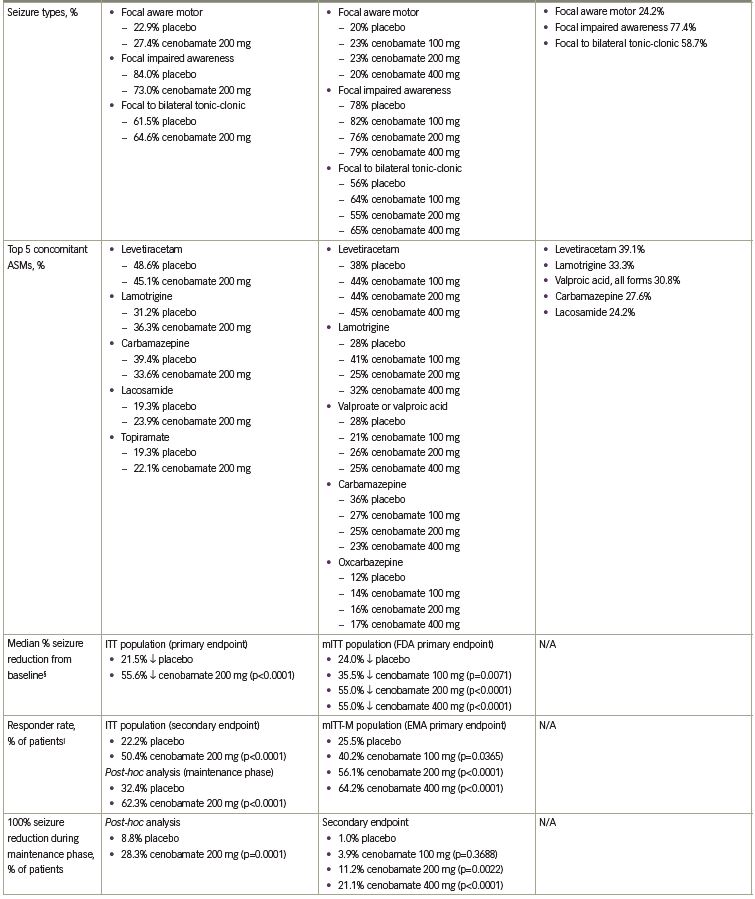
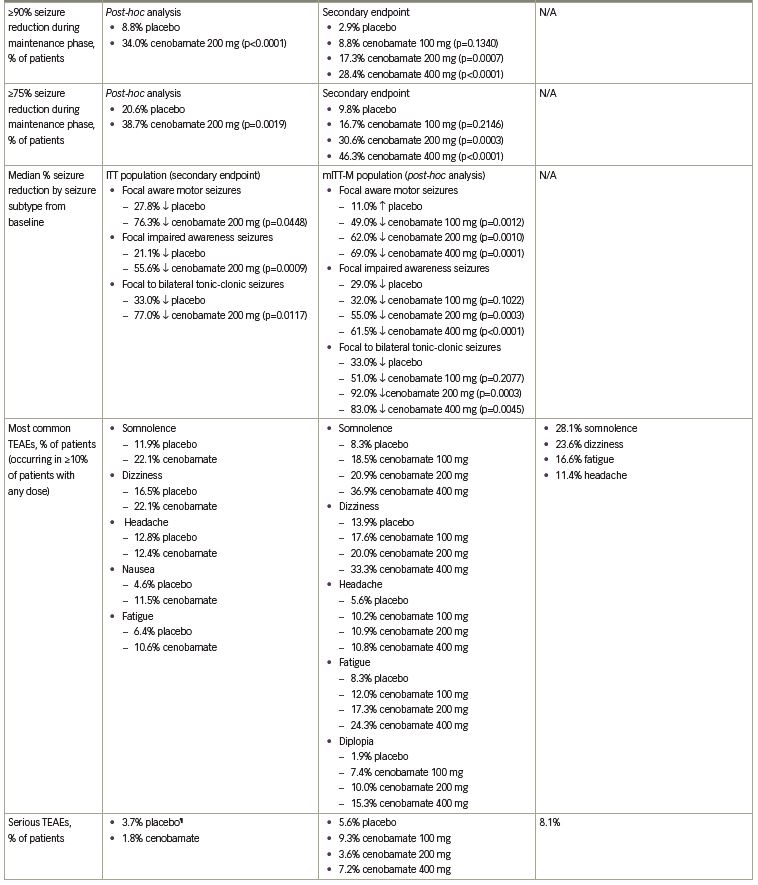
The primary endpoint was the median percent reduction in seizure frequency during the double-blind treatment period (titration and maintenance phases) in the intention-to-treat (ITT) population. The cenobamate group had a 55.6% median reduction in seizure frequency as compared with 21.5% in the placebo group (p<0.0001) (Table 1). The difference between cenobamate- and placebo-treated patients remained significant when assessing the secondary endpoint of median percent reduction for each focal seizure subtype. The median percent reduction in seizure frequency for specific types of focal seizures are listed in Table 1. Patients receiving cenobamate had a 50% responder rate during the double-blind period of 50.4%, while placebo-treated patients had a responder rate of 22.2% (p<0.0001) (Table 1). Cenobamate-treated patients were four times more likely than placebo-treated patients to have a ≥50% responder rate (odds ratio 3.94; 95% confidence interval [CI]: 2.14–7.24). A post-hoc analysis demonstrated that during the maintenance phase, there were significant differences in the ≥50%, ≥75%, ≥90%, and 100% seizure reduction rates between cenobamate- and placebo-treated patients, with approximately 20% more cenobamate-treated patients than placebo-treated patients experiencing seizure freedom (Table 1) (data on file, SK Life Science). In a post-hoc analysis of the completer population, cenobamate-treated patients experienced a substantial median percent reduction in seizure frequency (40.6% decrease from baseline versus 14.3% for placebo, p=0.001) during the first 4 weeks of the titration phase, while going from 50 to 100 mg/day. Cenobamate-treated patients continued to have further reductions in seizure frequency over the course of the study.26
Following completion of the double-blind treatment period in C013, patients from certain study sites were given the option to continue therapy in an open-label extension (OLE). Of 158 patients who were eligible, 149 (94.3%) entered the OLE (76 had received cenobamate and 73 had received placebo in the double-blind treatment period). All OLE participants were initated on cenobamate 100 mg/day with subsequent dose increases of 50 mg every 2 weeks as tolerated, with a maximum dose of 400 mg/day (with or without a taper of the double-blind study drug prior to open-label conversion in order to minimize the potential for triggering seizure exacerabations in patients with a clinically meaningful response [data on file, SK Life Science]). At the time of data cut-off, in April 2018, the median modal daily cenobamate dose was 200 mg/day and the median duration of cenobamate exposure was 60.6 months. The retention rate was 71% after 1 year and 65% after 2 years. A total of 60 patients discontinued the OLE: 28 due to withdrawal by the patient, 14 due to adverse events, 4 were lost to follow-up, 1 due to pregnancy, and 13 reported “other” as the reason for discontinuing the OLE.27 No patients discontinued the OLE due to lack of efficacy (data on file, SK Life Science).
In a second, larger dose-response study, C017 (NCT01866111), 437 adults with uncontrolled focal (partial-onset) seizures, despite ongoing therapy with one to three ASMs, were randomized 1:1:1:1 to cenobamate, titrated to maintenance doses of 100, 200, or 400 mg/day, or placebo for 18 weeks, including 6-week titration and 12-week maintenance phases. Levetiracetam, lamotrigine, valproate or valproic acid, carbamazepine and oxcarbazepine were the five most common concomitant ASMs across all groups. The initial cenobamate starting dose of the original titration schedule was 100 mg once daily for 1 week with 100-mg increments each week until the target maintenance dose was reached. An amendment to the protocol was made after a blinded review of the first nine patients because of treatment-emergent adverse events (TEAEs).28 Given the evidence that lower starting doses and slower up-titrations may improve tolerability,10,29 the starting dose for cenobamate was adjusted to 50 mg once daily for 1 week with a slower up-titration of 50 mg each week up to 200 mg/day, and then increased by 100 mg per week up to 400 mg/day.28 Forty-six patients were enrolled in the study before the protocol was amended (data on file, SK Life Science). Of note, in both the original and amended protocol, the 100 and
50 mg/day starting doses were higher and the weekly up-titrations were more rapid than is currently recommended.17
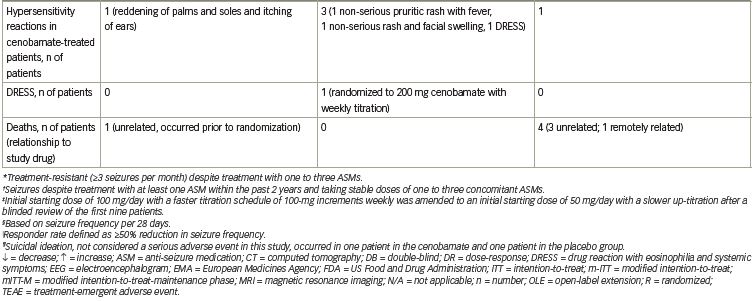
The primary endpoint for approval in the USA was median percent reduction in seizure frequency during the double-blind treatment period (titration and maintenance phases) in the modified ITT (mITT) population (all randomly assigned patients who had taken at least one dose of the study drug and had any post-baseline seizure data). There was a statistically significant median percent reduction in seizure frequency in all cenobamate dose groups versus placebo. A 24.0% reduction occurred in the placebo group versus a 35.5% reduction with cenobamate 100 mg/day (p=0.0071) and a 55.0% reduction with cenobamate 200 mg/day (p<0.0001) and 400 mg/day (p<0.0001) (Figure 1a). The responder rate (≥50% reduction in seizure frequency) in the mITT-maintenance (mITT-M) population (patients who completed the 6-week titration phase, took at least one dose of study medication, and had seizure data during the maintenance phase) was the primary endpoint for approval in the EU. The responder rate increased with increasing cenobamate doses (Figure 1b).28
Secondary endpoints included the median percent reduction in seizure frequency in the mITT-M population, the responder rate in the mITT population and the ≥75%, ≥90%, and 100% seizure reduction rates in the mITT-M population. A dose-response was seen in the maintenance phase for the median percent reduction in seizure frequency: 27.0% decrease with placebo versus 41.5% decrease with cenobamate 100 mg/day, p=0.0537; 56.5% decrease with cenobamate 200 mg/day, p<0.0001; and 63.0% decrease with cenobamate 400 mg/day, p<0.0001. The responder rates for the double-blind treatment period in the mITT population were analogous to those seen during the maintenance phase: 21.7% placebo versus 40.7% cenobamate 100 mg/day, p=0.0032; 57.8% cenobamate 200 mg/day, p<0.0001; and 60.4% cenobamate 400 mg/day, p<0.0001 (data on file, SK Life Science). Statistically significant seizure reduction rates of ≥75%, ≥90%, and 100% occurred in patients treated with cenobamate 200 and 400 mg/day as compared to placebo-treated patients in the mITT-M population. Seizure freedom was achieved by 11.2% and 21.1% of patients treated with cenobamate 200 and 400 mg/day compared with 1% of placebo-treated patients (Figure 1c, Table 1).28
Additional analyses evaluated outcomes based on focal seizure subtypes during the maintenance phase. The median percent reduction in seizure frequency by seizure subtype was a secondary endpoint (p-values assessed post-hoc). Statistically significant reductions in seizure frequency occurred in all seizure subtypes with cenobamate 200 and 400 mg/day in the mITT-M population (Table 1). Based on post-hoc analyses, the ≥50% responder rates for all seizure subtypes in all cenobamate dose groups ranged between 43.2% and 78.1%, while in placebo-treated patients, the responder rates ranged between 11.8% and 48.8%. Up to 30.0% of patients with focal aware (simple partial) motor seizures, 25.6% with focal impaired awareness (complex partial) seizures, and 52.8% with focal to bilateral tonic-clonic (secondarily generalized tonic-clonic) seizures experienced seizure freedom in the mITT-M population.28
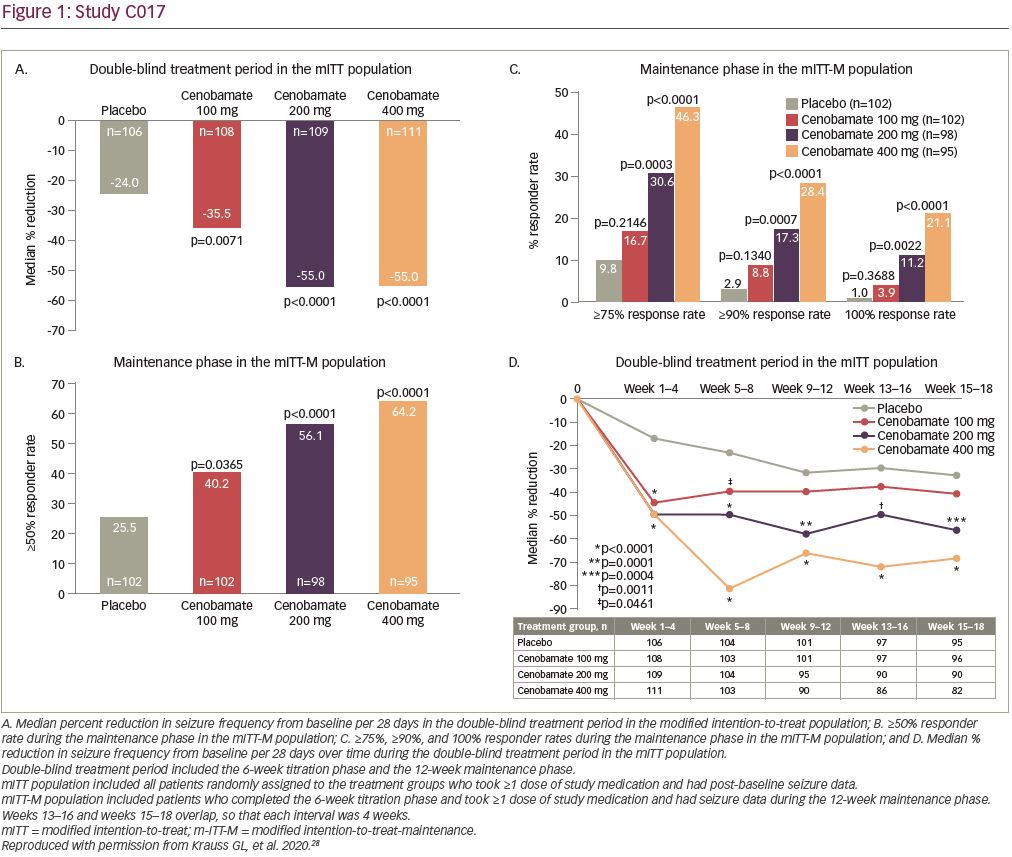
In a post-hoc analysis of the mITT population in the double-blind treatment period there was an approximate 50% median percent reduction in seizure frequency during the first 4 weeks of treatment with all cenobamate dose groups (compared with 17% in the placebo group), while titrating from 50 mg/day to the target dose of 100 or 200 mg/day. The 200 and 400 mg/day cenobamate groups continued to have further reductions over the course of the study (Figure 1d). The seizure-free rate in the first 4 weeks of the double-blind treatment period was approximately 6% in the cenobamate 100 mg/day group and 11% in both the cenobamate 200 and 400 mg/day groups, compared with 1% of placebo-treated patients. The seizure-free rate reached about 7%, 20%, and 28% in the cenobamate 100, 200, and 400 mg/day groups, respectively, and 7% in the placebo group during study weeks 9–12 (Figure 2) (data on file, SK Life Science).28
Additional post-hoc analyses evaluated the effects of various baseline features on the median percent reduction in focal seizures and responder rates with adjunctive cenobamate in C017. Cenobamate-treated patients experienced clinically relevant reductions in median seizure frequency as compared with placebo, regardless of the severity of disease, which was defined by the number of baseline ASMs (1, 2, or >2), the number of median baseline siezures per 28 days (≤9.5, >9.5), and the median baseline epilepsy duration in years (≤23, >23). Cenobamate-treated patients also generally had higher responder (≥50%) and seizure freedom rates regardless of the severity of disease versus placebo-treated patients.30
After patients completed the entire double-blind treatment period in C017 they were given the option to enroll in the C017 OLE. A total of 355 out of 360 (98.6%) patients who completed the double-blind treatment period elected to participate in the OLE (265 had received cenobamate and 90 had received placebo in the double-blind treatment period). Patients were converted to a target cenobamate dose of 300 mg/day over a 2-week blinded period. At the time of data cut-off, in April 2018, patients received a median modal dose of 300 mg/day of cenobamate for a median of 40 months. After 1 and 2 years, the retention of patients in the OLE was 80% and 70%, respectively. A total of 125 patients discontinued the OLE, including 55 patients due to lack of efficacy, 24 due to adverse events, 23 due to withdrawal by the patient, 7 were lost to follow-up, 5 had died, 2 had protocol violations, and 9 reported “other” as the reason for discontinuing the OLE. The median reduction in seizure frequency was 65.4% in all OLE patients in months 1–6. Patients treated with cenobamate during the double-blind period who entered the OLE and continued to receive cenobamate, and patients treated with placebo during the double-blind period who entered the OLE and were switched to cenobamate, had similar median reductions in seizure frequency (65.7% and 63.0%, respectively) at this time point. Further median reductions occurred over time in all groups. Similarly, ≥50% responder rates increased over time in all OLE patients, reaching 74.9% in months 25–30. Seizure freedom was achieved in 20.2% (45/223) of evaluable OLE patients at 25–30 months. Among the 45 patients who were seizure-free at months 25–30, the median duration of seizure freedom achieved during the entire OLE was 33.2 months (range 13.2–50.4 months).31
Cenobamate 100, 200, and 400 mg/day produced clinically relevant median percent reductions in seizure frequency in adults with uncontrolled focal epilepsy.26,28 During the double-blind treatment period the median percent reduction in seizure frequency in both studies ranged from 35.5% (cenobamate 100 mg/day in C017) to approximately 55% (cenobamate 200 mg/day in C013 and cenobamate 200 and 400 mg/day in C017), with greater reductions seen with cenobamate 200 and 400 mg/day.26,28 During the maintenance phase in C017, the reduction in seizure frequency reached 63.0% with cenobamate 400 mg/day.28 Approximately ≥50% of patients treated with cenobamate ≥200 mg/day in both studies had a ≥50% reduction in seizure frequency in the double-blind period, with the responder rate in C017 ranging from 40.2% (cenobamate 100 mg/day) to 64.2% (cenobamate 400 mg/day) in the maintenance phase.26,28 During the maintenance phase in C013 (cenobamate 200 mg/day) and C017 (cenobamate 400 mg/day), the difference between cenobamate- and placebo-treated patients in achieving seizure freedom was 20%.26,28 Cenobamate decreased seizure frequency in all focal seizure subtypes.26,28 In both studies a 40–50% median reduction in seizure frequency occurred in the first 4 weeks of titration from a dose of 50 mg/day to the target maintenance dose, and further reductions occurred in patients treated with cenobamate 200 (C013, C017) and 400 mg/day (C017) over time.26,28 In particular for C017, patients achieved seizure freedom in the first 4 weeks of cenobamate treatment as the dose was being titrated from the starting dose of 50 mg/day to target maintenance doses of 100 or 200 mg/day, with an increase in the seizure-free rate over time in all cenobamate dose groups.28 The C017 OLE confirmed that seizure reduction was maintained over a prolonged duration of time.31
Cenobamate safety
Three clinical studies (C013, C017, C021) have evaluated the safety of cenobamate. Somnolence, dizziness, headache, nausea, and fatigue occurred in ≥10% of cenobamate-treated patients in C013 (Table 1). The majority of TEAEs (74.3%) were mild or moderate in severity. Five patients discontinued cenobamate due to eight TEAEs (tachycardia, abdominal pain, gastroesophageal reflux, drug hypersensitivity, nystagmus, aggression, depression, dyspnea) (data on file, SK Life Science). No cases of drug reaction with eosinophilia and systemic symptoms (DRESS) occurred.26 Similarly, in C017 the TEAEs that occurred in ≥10% of cenobamate-treated patients were CNS-related (somnolence, dizziness, headache, fatigue) along with diplopia (Table 1). Mild or moderate TEAEs occurred in 54.6% of patients treated with cenobamate 100 mg/day, 66.4% of patients treated with cenobamate 200 mg/day, and 73.9% of patients treated with cenobamate 400 mg/day. TEAEs that led to treatment discontinuation in cenobamate-treated patients occurred in 10.2% of patients who received 100 mg/day, 13.6% of patients who received 200 mg/day, and 19.8% of patients who received 400 mg/day. The TEAEs that led to ≥1 patient discontinuing in any group included ataxia, somnolence, dizziness, nystagmus, and vertigo. There was one case of DRESS.28 A post-hoc analysis of the data from C013 and C017 found that CNS-related events and TEAEs that led to treatment discontinuation occurred most often during the titration phase.32 Of note, dose adjustment of concomitant ASMs was not allowed during the double-blind treatment periods in C013 and C017.26,28
C021 (NCT02535091) is a phase III, open-label, ongoing long-term (12-month) safety study in adults with uncontrolled focal (partial-onset) seizures.33 Unlike the efficacy studies, patients taking concomitant phenytoin and phenobarbital were included in C021 for a PK analysis to evaluate the impact of cenobamate on their plasma concentrations (reviewed below in the drug–drug interactions section). As prior studies identified three cases of DRESS (two in phase I studies with one fatality, one in C017) in patients who used a titration schedule faster than every 2 weeks and a starting dose of ≥50 mg/day of cenobamate (data on file, SK Life Science),28,33 C021 specifically utilized a titration schedule with a lower starting dose (12.5 mg/day) and a slower titration rate (every 2 weeks) to minimize the risk of DRESS.33,34 Cenobamate was titrated over 12 weeks to an initial target dose of 200 mg/day (12.5, 25, 50, 100, 150, and 200 mg/day at 2-week intervals), with increases to a maximum of 400 mg/day (using biweekly increments of 50 mg/day) permitted if clinically warranted. Cenobamate exposure was ≥6 months for 82.9% (1,110/1,339 patients) of the patients at the time of the interim analysis. Levetiracetam, lamotrigine, all forms of valproic acid, carbamazepine, and lacosamide made up the five most common concomitant ASMs, with only 8.5% and 3.8% of patients taking concomitant phenytoin and phenobarbital, respectively. TEAEs that occurred in ≥10% of patients were somnolence, dizziness, fatigue, and headache (Table 1). Most (77.8%) of the TEAEs were mild or moderate in severity. TEAEs related to nervous system disorders (3.4%) and skin and subcutaneous tissue disorders (3.3%) were the most common TEAEs leading to cenobamate discontinuation. There were no cases of DRESS.33
The most frequent TEAEs during the double-blind periods in C013 and C017, and in the open-label C021 study, were CNS-related events, with higher rates reported with cenobamate 400 mg/day as compared with 100 and 200 mg/day in C017 (Table 1). The majority of TEAEs in cenobamate-treated patients were mild or moderate and discontinuation rates due to TEAEs were low. Of these three clinical studies, one case of DRESS occurred in C017 during the original protocol, which started with an initial dose of 100 mg/day and titrated up by 100 mg weekly (the protocol was later amended to decrease the starting dose to 50 mg/day and use a slower titration to improve tolerability) (Table 1). No cases of DRESS occurred in C013 or C021, where initial doses of 50 mg/day (not the recommended starting dose) and 12.5 mg/day were used, respectively, coupled with titration every 2 weeks.
Drug–drug interactions
Cenobamate exposure is marginally impacted by other ASMs. Co-administration with phenobarbital, carbamazepine, or valproate led to minimal change in cenobamate multiple-dose exposure. Cenobamate Cmax and AUC decreased by 27% and 28%, respectively, when cenobamate was given concurrently with phenytoin.17
In vivo drug–drug interaction studies demonstrated that cenobamate is an inducer of CYP2B6 and CYP3A4 and inhibitor of CYP2C19.17,35,36 A CYP450 probe study found that cenobamate induces CYP2B6 (bupropion probe) and has dose-dependent CYP3A4 (midazolam probe) induction, with 200 mg of cenobamate having a greater impact on midazolam Cmax and AUC than 100 mg of cenobamate. The probe study also established that cenobamate inhibits CYP2C19 (omeprazole probe) and has no effect on CYP2C9 (warfarin probe).36
Certain ASMs may need to be dose adjusted when used concomitantly with cenobamate. Based on population PK analyses, concurrent administration of cenobamate is expected to decrease lamotrigine plasma concentrations by 21–52%, suggesting an increase in the lamotrigine dose may need to be considered.17 However, data from a subset of study sites in the C021 study indicated that the mean dose of lamotrigine was actually reduced by investigators due to pharmacodynamic considerations when used concomitantly with cenobamate (data on file, SK Life Science). Mean carbamazepine Cmax and AUC were both shown to decrease by 23% with co-administration of cenobamate due to CYP3A4 induction.11,35 In contrast, cenobamate’s inhibition of CYP2C19 resulted in increased plasma concentrations of phenytoin and phenobarbital when these medications were co-administered with cenobamate.11,35 Cenobamate increased mean phenytoin Cmax and AUC by 70% and 84%, respectively, and mean phenobarbital Cmax and AUC by 34% and 37%.17 Increased concentrations of the active metabolite of clobazam, N-desmethylclobazam, are also expected to occur due to CYP2C19 inhibition.11,17 Dose reductions of these three ASMs may be warranted when they are taken with cenobamate to minimize the risk of their adverse effects. For phenytoin, doses need to be titrated down by as much as 50% as cenobamate is being titrated up.17 A PK evaluation from the C021 study suggested that reduction of the phenytoin dose may need to begin when cenobamate is titrated from 25 to 50 mg/day. In this study, 43.4% and 29.7% of patients taking phenytoin and phenobarbital had their doses decreased. Periodic reductions in phenytoin and phenobarbital doses of 25–33% (by no more than up to two-thirds the total baseline dose), based on adverse events, plasma concentrations, and/or clinical discretion, resulted in relatively stable mean plasma concentrations of both drugs during cenobamate titration.33
For non-ASM medications, doses of CYP2B6 substrates (e.g., efavirenz)37 and CYP3A substrates (e.g., benzodiazepines, protease inhibitors, statins)17,37 may need to be increased to maintain their efficacy as co-administration with cenobamate may decrease their plasma concentrations.17 Doses of CYP2C19 substrates (e.g., omeprazole and voriconazole)37 may need to be decreased to reduce the risk of adverse effects when they are used with cenobamate as concomitant administration increases their plasma concentrations.17 Women taking oral contraceptives and cenobamate simultaneously should use additional or alternative non-hormonal birth control as cenobamate may decrease plasma concentrations of contraceptive steroids.17
Cenobamate dosing
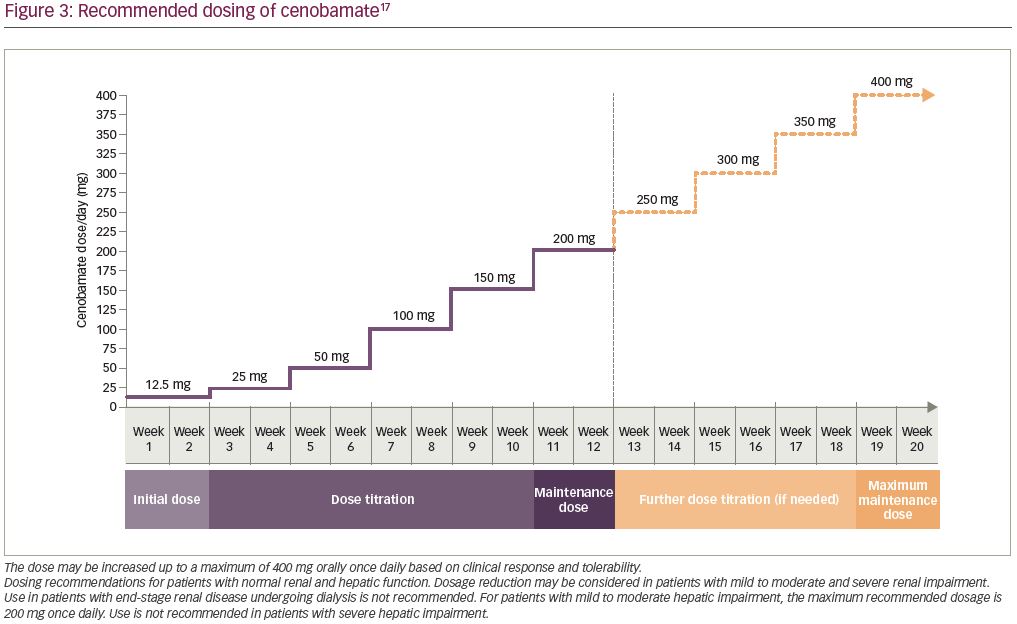
Based on the C021 study, the recommended dosing in the XCOPRI prescribing information reflects the initial dose and titration schedule from that study. Increasing daily doses of cenobamate are administered (12.5, 25, 50, 100, 150, and 200 mg) at 2-week intervals. Further dose escalation may be warranted after at least 2 weeks of treatment with 200 mg/day, based on clinical response and tolerability, in which case cenobamate can be increased by 50 mg once daily every 2 weeks, up to a maximum maintenance dose of 400 mg/day (Figure 3). When discontinuing cenobamate the dose should be down-titrated over a period of ≥2 weeks; if safety concerns require abrupt withdrawal, the potential risks of abrupt discontinuation may be mitigated by cenobamate’s long half-life.17
Overall summary
Two pivotal studies, in similar patient populations and using different cenobamate titration schedules, have demonstrated the efficacy of adjunctive cenobamate in adults with uncontrolled focal epilepsy despite treatment with one to three ASMs at doses of 100, 200, and 400 mg/day. Cenobamate reduced the frequency of focal (partial-onset) seizures by approximately 55% from baseline, with some patients experiencing greater reductions with higher doses. Reductions in seizure frequency may occur with doses as low as 50 mg/day during titration, prior to patients reaching the cenobamate maintenance dose of ≥200 mg/day, up to a maximum dose of 400 mg/day. The seizure freedom rates are encouraging and higher than previously reported with other adjunctive ASMs in uncontrolled focal epilepsy, with up to 20% more cenobamate-treated patients, varying by dose, compared with placebo-treated patients achieving seizure freedom with adjunctive cenobamate.38,39 However, comparisons to results from other studies have limitations due to differences in study designs and inconsistent methods for analyzing seizure freedom. In line with other randomized, placebo-controlled studies of adjunctive ASMs, a number of placebo-treated patients in the C013 and C017 studies had reductions in seizure frequency or achieved seizure freedom.39–41 This phenomenon of “placebo response” is not exclusive to the adjunctive cenobamate clinical studies and has been documented in other adjunctive ASM studies, whereby patients in both the placebo and study-drug groups are taking background ASMs, to be approximately 20% seizure free.42 The “placebo response” is suggested to be influenced by study design, patient population, patient expectations, duration of the study, and patient–investigator interaction during clinical trials.42,43 Cenobamate is generally safe, with CNS-related side effects being the most commonly reported TEAEs. Although three cases of DRESS have been reported, no cases were reported with the dosing regimen used in the C021 study, which is the regimen recommended in the XCOPRI prescribing information (i.e., starting with a low dose of 12.5 mg/day and increasing the dose slowly every 2 weeks). Altogether, the data suggest that adjunctive cenobamate shows great promise as a treatment option for patients with uncontrolled focal epilepsy.