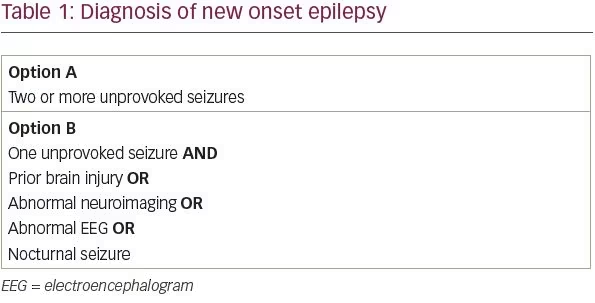
The International League Against Epilepsy (ILAE) revised its definition of epilepsy in 2014 in order to maximize early identification and treatment of patients with epilepsy.1 The ILAE’s conceptual definition of epilepsy, first formulated in 2005, is “a disorder of the brain characterized by an enduring predisposition to generate epileptic seizures.” In practice, this definition corresponded to patients with two or more unprovoked seizures more than 24 hours apart. We know, however, that not all patients with a single unprovoked seizure are equally likely to have a second seizure. Under the new ILAE definition, patients with a single unprovoked seizure and a likelihood of recurrent seizure above 60% now meet criteria for a diagnosis of epilepsy.
In a systematic review of studies assessing the risk of seizure recurrence following a single unprovoked seizure, between 21 and 45% of patients had another unprovoked seizure within the first 2 years.2 From a practical standpoint, four risk factors have been identified that approximately double a patient’s risk of seizure recurrence: a prior brain injury, an abnormal neuroimaging finding that corresponds to the suspected seizure focus, focal or generalized epileptiform discharges on electroencephalogram (EEG), or a nocturnal seizure.3–6 Thus, patients with a single unprovoked seizure and at least one of these four risk factors likely has a greater than 60% chance of recurrent seizure and can be diagnosed with epilepsy (Table 1).
Although a major early study suggested that an abnormal neurologic examination was associated with an increased risk of seizure recurrence, a follow-up study from the same group found no increase in risk.3,7 The same follow-up study found that having a sibling with epilepsy increases the risk of recurrence among patients with idiopathic epilepsy, but even among these patients, the recurrence risk is only 46% at 5 years. Thus, neurologic examination findings and family history of epilepsy should be taken into consideration when evaluating a patient with a first seizure, but these features alone are not sufficient to make a diagnosis of epilepsy.
Consideration of antiepileptic drug treatment in new onset epilepsy
Most early studies of seizure recurrence after an initial unprovoked seizure included both antiepileptic drug (AED)-treated and untreated patients, making it difficult to estimate the true risk of recurrent seizure. The FIRST study found that 51% of patients who did not start an AED after a first seizure had another one within 2 years, while only 25% of those who started an AED had another seizure within the same period.8 However, later studies have shown that although AED treatment does reduce the risk of seizure recurrence within the first 2 years after an initial seizure, it does not affect the likelihood of long-term seizure remission or quality of life.9,10 As early AED treatment is not guaranteed to change a patient’s overall outcome, and AEDs are not entirely benign medications, not all patients who are diagnosed with epilepsy choose to start treatment right away.
All decisions about AED treatment should be made in collaboration with the patient after a careful discussion of the risks and benefits, weighing the risk of harm due to seizures against the potential adverse effects of AEDs. The presence of a seizure type that is associated with a higher risk of injury or death, such as tonic-clonic seizures or status epilepticus, may be one factor influencing the patient and physician’s decision of whether to start AEDs.11–13
Characterization of epilepsy type
Determination of the patient’s epilepsy type—focal or generalized—at the time of initial diagnosis is important because it helps predict prognosis and guide selection of an appropriate AED. This determination is typically made based on seizure semiology along with magnetic resonance imaging (MRI) and EEG findings. Lateralized motor or sensory symptoms, forced eye deviation or head turn, automatisms, language disturbances, and experiential phenomena suggest focal onset, while bilateral myoclonic jerking or initial bilateral tonic activity suggest generalized onset.
However, semiology alone can be misleading: focal seizures may lack lateralizing features at onset, and more than half of patients with generalized epilepsy have focal seizure symptoms.14 Moreover, three-quarters of patients with focal epilepsy are amnestic for at least some of their seizures, and 30% are amnestic for all seizures.15 Additionally, up to 60% of patients do not have an aura preceding their seizures.16 These factors make the diagnosis and characterization of epilepsy challenging in many patients.
Most patients with a first seizure should have an MRI, unless there is a contraindication. MRI has a higher yield than computed tomography (CT) for detecting focal epileptogenic lesions.17–19 The presence of a focal lesion can confirm a focal onset if the lesion’s location corresponds to the patient’s semiology. For patients with a clear electroclinical primary generalized epilepsy syndrome, such as juvenile myoclonic epilepsy, neuroimaging may not be required.
If an MRI is obtained, recommended sequences include a 3D T1 sequence with 1 mm isotropic slices (e.g., magnetization-prepared rapid gradient echo [MPRAGE]), axial and coronal T2 and FLAIR sequences with ≤3 mm slices, and an axial T2* sequence for hemosiderin and calcification (e.g., susceptibility-weighted imaging [SWI] or gradient echo [GRE]).20 Contrast is not required for all patients, but should be considered for patients over 50 years of age, due to the higher likelihood of metastatic or primary brain tumor in this age group. When possible, 3 Tesla MRI is preferred over 1.5 Tesla as it is more sensitive for epileptogenic lesions.21,22 Recently, 7 Tesla MRI scanners have increased sensitivity even further, but these are typically only available through research protocols.23
EEG is the other essential modality for diagnosis and characterization of new onset epilepsy. A single routine EEG may be insufficient for the detection of epileptiform abnormalities; a recent meta-analysis of 15 studies found that the sensitivity of a routine EEG was 17%.24 In order to increase this yield, physicians should consider performing an extended 60-minute EEG, a sleep-deprived EEG, or up to three serial routine EEGs.25–27 Ambulatory EEG recordings also increase the likelihood of detecting epileptiform abnormalities; the benefits of prolonged recording diminish after 48 hours, as 95% of abnormalities are detected within this time frame.28 An inpatient video EEG study may be considered in specific scenarios, such as differentiating epileptic seizures from psychogenic non-epileptic attacks.29
Selecting an antiepileptic drug—focal epilepsy
The SANAD trial—first-line treatment of new onset focal epilepsy
The goal of AED treatment in new onset epilepsy is to control seizures with a single AED while minimizing adverse effects. Although there are many drugs available for the treatment of focal epilepsy, there are few controlled studies comparing their efficacy and tolerability. The SANAD trial, published in 2007, was a seminal study of 1,721 patients with focal epilepsy randomized to treatment with carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate.30 The primary outcome was time to treatment failure, defined as discontinuation of the drug due to uncontrolled seizures or adverse effects. Lamotrigine was significantly better than carbamazepine, gabapentin, and topiramate, and non-significantly better than oxcarbazepine. For time to 12-month remission, however, carbamazepine was significantly better than gabapentin and non-significantly better than lamotrigine, topiramate, and oxcarbazepine.30
The results of the SANAD trial suggest that lamotrigine is the best first-line treatment for focal epilepsy due to its combination of efficacy and tolerability, with carbamazepine as a reasonable alternative in patients who are able to tolerate it.
Newer antiepileptic drugs for first-line treatment of focal epilepsy
Since the publication of the SANAD trial, several new focal epilepsy drugs have entered the market. While all of these drugs were initially studied as adjunctive treatments, many are now used as monotherapy, and some, particularly levetiracetam, are now commonly used as first-line treatment. A major advantage of these newer drugs is that the doses can be increased quickly in patients with frequent seizures, without the risk of Stevens–Johnson syndrome, a known adverse effect of lamotrigine, and to a lesser extent carbamazepine.
Among the new AEDs, four—levetiracetam, zonisamide, lacosamide, and eslicarbazepine—have been compared with older AEDs in randomized controlled trials, as shown in Table 2.31–41 Trials were identified using PubMed’s clinical trials filter and the search terms “monotherapy,” and “[drug name]”, and were included if they studied adult patients with focal epilepsy comparing a newer AED to one of the standard AEDs (those included in the SANAD trial).
Of the four new AEDs, levetiracetam is the best studied and most widely prescribed; it has largely become the default first-line AED for new onset epilepsy due to its ease of titration and favorable side effect profile. Levetiracetam is now the most commonly prescribed first-line AED in older adults, accounting for 45.5% of prescriptions.42 Studies have shown that levetiracetam has similar efficacy compared to older AEDs, though it was inferior to carbamazepine in a subgroup analysis of one study (Table 2).
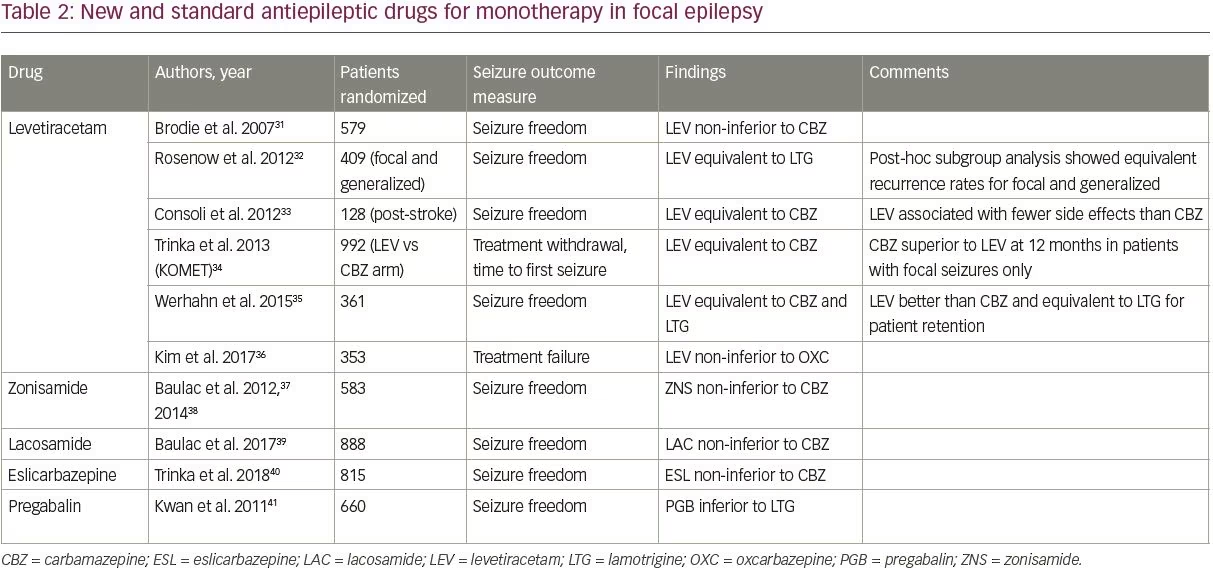
Importantly, only two studies compared levetiracetam to lamotrigine; both studies found similar efficacy for the two drugs.32,35 The focal epilepsy arm of the SANAD II trial, which will be the largest study to compare the long-term efficacy and tolerability of levetiracetam and lamotrigine, is currently underway (ISRCTN30294119).
Outside of levetiracetam, high-quality data assessing the new AEDs as monotherapy is limited. Zonisamide, lacosamide, and eslicarbazepine have all been shown to be non-inferior to carbamazepine in a single study each (Table 2); zonisamide is also being studied in the focal arm of the SANAD II trial. These three agents can be considered as options for first-line treatment depending on the patient’s comorbidities and contraindications. Pregabalin has also been studied in comparison with lamotrigine and was inferior,41 and thus is not recommended as first-line treatment.
Brivaracetam was well-tolerated in two randomized studies of conversion from polytherapy to monotherapy, but the number of patients remaining on brivaracetam at the end of these studies was too low to determine its efficacy as monotherapy;43 it has also not been studied in direct comparison with older AEDs. Thus, there is insufficient evidence to recommend brivaracetam monotherapy as first-line treatment at this time.
Clobazam, perampanel, and cenobamate are new AEDs with the potential for use as monotherapy. Both clobazam and perampanel were effective and well-tolerated in small retrospective studies that included patients with both focal and generalized epilepsy.44,45 Clobazam was also superior to carbamazepine and phenytoin in a randomized trial of pediatric patients, but has not been directly compared to the older AEDs in an adult population.46 Cenobamate, an enhancer of fast and slow sodium channel inactivation with once-daily dosing, recently demonstrated efficacy as an adjunctive agent for patients with focal epilepsy, but has not yet been studied as monotherapy.47
Summary of first-line treatment for focal epilepsy
Levetiracetam has performed as well as, or slightly worse than, older AEDs in head-to-head trials,31–36 and is a reasonable first-line treatment in patients without a history of psychiatric issues, particularly if seizures are frequent or patients have difficulty with the lamotrigine titration schedule. In patients with psychiatric comorbidities, we recommend lamotrigine as first-line treatment. The pending SANAD II trial will more definitively answer the question of which of these two AEDs is superior with regards to both efficacy and tolerability. Depending on the patient’s comorbidities and side effect tolerance, several of the older AEDs (carbamazepine, oxcarbazepine, topiramate) and newer AEDs (zonisamide, lacosamide, eslicarbazepine) may be reasonable alternatives. Brivaracetam, clobazam, perampanel, and cenobamate may be viable options in the future, but there is insufficient evidence at this time. Gabapentin and pregabalin should not be used as first-line treatments.
Selecting an antiepileptic drug—generalized or unclassified epilepsy
The SANAD trial—first-line treatment of new onset generalized epilepsy
The 2007 SANAD trial included a second arm, which studied patients with generalized epilepsy or epilepsy that could not be definitively classified at the time of treatment initiation.48 Valproate was significantly better than topiramate for time to treatment failure in the overall analysis, and significantly better than both topiramate and lamotrigine in patients with a confirmed diagnosis of generalized epilepsy. For time to 12-month remission, valproate was significantly better than lamotrigine in both groups, but not significantly different from topiramate in either group. Thus, valproate appeared to have the best combination of efficacy and tolerability of the three drugs studied.
Despite valproate’s success in SANAD, its use outside of the trial setting is problematic because of its adverse effects. Among women of childbearing age, valproate is contraindicated due to teratogenicity, as it significantly increases the risk of congenital malformations and long-term neurocognitive deficits.49–52 As of 2018, the European Union has banned the use of valproate in women of childbearing age unless they are enrolled in a pregnancy prevention program.53 Valproate also has several other undesirable side effects including weight gain, hair loss, polycystic ovarian syndrome, and hepatic encephalopathy.54–57 Alternative first-line treatments are needed, especially for women of childbearing age.
Newer alternatives to valproate for the treatment of generalized epilepsy
Levetiracetam is the most extensively studied new AED for generalized epilepsy. Two of the monotherapy trials described above also included patients with generalized epilepsy.32,34 In the KOMET study, levetiracetam was equivalent to valproate for both treatment withdrawal rate and time to first seizure.34 Levetiracetam has also been shown to be equivalent to lamotrigine for seizure freedom rate in a heterogenous sample; post-hoc subgroup analysis showed that this was true for both focal and generalized epilepsy.32
Recently, Marson and colleagues presented the results of the generalized epilepsy arm of the SANAD II trial.58 Valproate was superior to levetiracetam for time to treatment failure, time to first seizure, and time to 24-month remission. Interestingly, the difference in time to treatment failure was attributable to inadequate seizure control, rather than to adverse effects, which suggests that valproate was not more poorly tolerated than levetiracetam in this sample.
As noted above, both clobazam and perampanel were shown to have good efficacy and tolerability in retrospective observational studies that included patients with generalized epilepsy, but neither drug has been compared with any of the older drugs in a randomized trial.44,45 To our knowledge, none of the other new AEDs have been studied as monotherapy in patients with generalized epilepsy.
Summary of first-line treatment for generalized and unclassified epilepsy
Although the SANAD II results suggest that valproate remains the most effective AED for generalized or unclassified epilepsy, we do not recommend it as first-line treatment due to its adverse effects. Lamotrigine is a good choice if seizure frequency is low enough to allow slow dose increases. If rapid onset is needed, levetiracetam can be used. If seizures are refractory to these two agents, valproate can be used after careful discussion of the risks and benefits.
Counseling patients with new onset epilepsy
Patients with a new diagnosis of epilepsy typically have many questions about the disease and its impact on their lives. The most frequent questions are about the long-term prognosis of epilepsy, whether to start AED treatment, and whether AED treatment will be lifelong. As discussed above, patients should be informed that immediate AED treatment reduces the risk of early seizure recurrence, but does not change the overall prognosis of epilepsy, and the decision about whether to start an AED should be made on an individualized basis. Patients should be informed that about half of patients will achieve seizure control with their first AED, while an additional 20% will respond to a second or third drug.59 Additionally, they should be aware that any attempts to discontinue AED treatment should not be initiated until after at least 2 years of seizure freedom, and that nearly half of patients will have a recurrence of seizures after AED withdrawal.60
Outside of seizure control, the most common practical questions relate to driving. Any seizure with impaired awareness—absence, tonic-clonic, or focal unaware—can cause significant harm if it occurs while the patient is driving. Most states require an interval of seizure freedom before a patient can resume driving. Physicians should ensure that patients are familiar with their state’s laws. Some states require physicians to report their patients with epilepsy or seizures to the Department of Motor Vehicles, while others do not.61 It is important to note that in states without mandatory reporting, physicians may not have legal protection when disclosing a diagnosis of epilepsy without the patient’s consent. In these cases, physicians will need to weigh the risks of the patient continuing to drive (particularly in the case of commercial drivers) against the risks of legal ramifications of violating the patient’s confidentiality. All conversations about driving should be documented in the patient’s medical record.
Women of childbearing age should additionally be counseled about the implications of epilepsy and AEDs on pregnancy. Patients should be aware that seizures, especially generalized tonic-clonic seizures, and some AEDs, can have adverse effects on a developing fetus. In women who are not planning pregnancy, long-acting contraception with an intrauterine device or depot injection is preferred as these minimize opportunity for user error. Patients should also know that treatment with enzyme-inducing AEDs can lower the efficacy of hormonal contraception.62 All women with epilepsy who are planning pregnancy should inform their neurologist ahead of time; if a pregnancy is unplanned, the neurologist should be informed as soon as possible. Additionally, all women with epilepsy of childbearing age should take folic acid whether or not a pregnancy is planned, as this reduces the risk of neural tube defects that develop early in the first trimester, often before a pregnancy is known.63
The most difficult topic to discuss with newly diagnosed patients is sudden unexpected death in epilepsy (SUDEP). Risk factors for SUDEP include high seizure frequency, early age of onset, long duration of disease, generalized tonic-clonic seizures, nocturnal seizures, living alone, male gender, alcohol dependence, and substance abuse.64–66 Most neurologists believe that all patients with epilepsy should be informed about SUDEP, while some argue that SUDEP counseling is only necessary for patients who are at high risk.67 We recommend discussing SUDEP with all patients with new onset epilepsy as part of an initial discussion about the importance of medication compliance, with more frequent and extensive counseling for high-risk patients.








