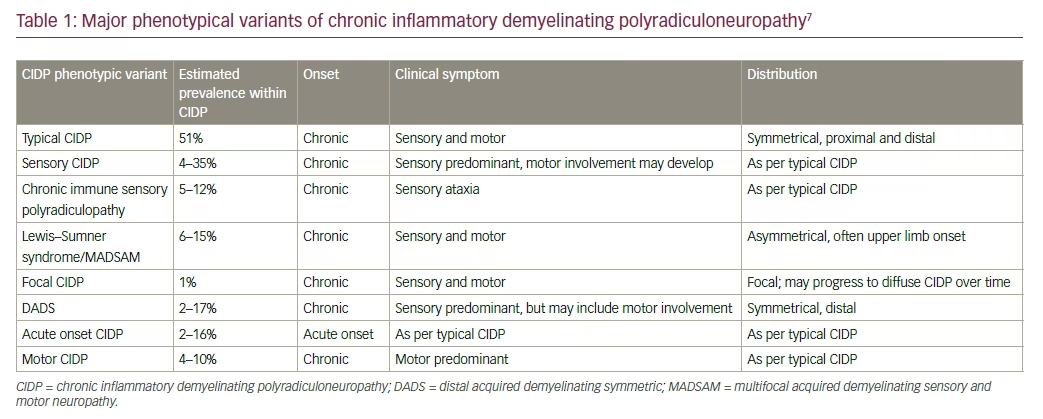
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is an autoimmune demyelinating polyradiculoneuropathy characterized by chronically progressive weakness and impaired sensory function in the lower and upper extremities.1 Symptoms, which are progressive over at least 8 weeks, may include weakness of the arms and legs (both proximal and distal), loss of vibration and joint position sense, poor balance, numbness, paresthesias, and loss of deep tendon reflexes (areflexia). Cranial nerves (other than cranial nerve V or VII) and autonomic functions are generally preserved.2 The phenotype of symmetrical proximal and distal motor and sensory symptoms and signs define typical CIDP. Atypical CIDP includes other clinical presentations, such as asymmetric, multifocal motor and sensory symptoms, distal sensory, or predominantly motor or sensory types. Moreover, up to 16% of patients with CIDP may demonstrate acute-onset CIDP, which is characterized by a rapidly progressive onset within 8 weeks.3,4
The exact mechanisms that underlie the development of CIDP have not been elucidated fully, although evidence suggests likely contributions by both cellular and humoral factors. It is twice as common in men, with increasing frequency after age 60, although it can occur at any age.5 The incidence and prevalence of CIDP have been estimated at 1.6/100,000/year to 8.9/100,000, respectively.6 There are many phenotypic variants of CIDP (Table 1), which suggests that the disorder may not be a discrete entity, but a spectrum of conditions.7 Elevated levels of cerebrospinal fluid (CSF) protein are present in the majority of patients although normal CSF results do not exclude the diagnosis of CIDP.7,8 Currently, there are no well-established biomarkers, although autoantibodies to contactin-1 and neurofascin-155 define CIDP subsets of patients with specific clinical features.9
Diagnosis
Early diagnosis is vital for this treatable condition in order to limit disability as a result of secondary axonal damage. Initial diagnostic criteria, including
the American Academy of Neurology and Inflammatory Neuropathy Cause and Treatment (INCAT) criteria, were designed for research and have a high specificity, but low sensitivity for CIDP. For this reason, many patients do not meet the diagnostic criteria and do not receive the appropriate treatment.10,11 More recently, diagnostic criteria for use in clinical practice have been developed, including the European Federation of Neurological Sciences (EFNS)/Peripheral Nerve Society (PNS), Neuropathy Association, and the Koski criteria. According to the EFNS/PNS criteria, CIDP should be considered in a patient if there is clinical evidence for a progressive symmetrical or asymmetrical polyradiculoneuropathy and a clinical course that is relapsing and remitting or progresses for >2 months.12 Electrodiagnostic testing is essential to make the diagnosis, EFNS/PNS electrodiagnostic criteria for definite or probable diagnosis of CIDP require the presence of demyelinating findings (DF) in at least 2 nerves; for possible CIDP, abnormality may need to be evident only in 1 nerve.10 The DF can include any of those abnormal parameters: Prolongation of distal motor latency (>50%), slowing of conduction velocity (<30%), absence or prolongation of F response latencies, presence of partial conduction block (50% for definite, 30% for probable), and abnormal temporal dispersion (>30% prolongation of CMAP duration between distal and proximal CMAP). Preliminary evidence suggests that more extensive testing such as 8 motor nerves13 or 3- rather than 2-limb testing may increase the diagnostic sensitivity for definite CIDP, particularly in individuals with atypical (asymmetric and distal) phenotypes, which comprised 75.5% (40 of 53) of the study cohort.14
With unilateral, forearm/foreleg, four-nerve studies the EFNS/PNS criteria has been reported to provide a sensitivity of 81.3% and specificity of 96.2% for “definite/probable” CIDP.15 Supportive criteria include an elevated CSF protein with leukocyte count <10/mm3 per high powered field, magnetic resonance imaging (MRI) of the nerve roots, nerve biopsy, and treatment response to immunomodulatory therapy. For diagnosis, objective measures should be used to verify and apply the criterion of treatment response. Electrodiagnostic studies including sensory and motor nerve conduction studies should be performed; studies may need to be performed bilaterally, or use proximal stimulation in motor nerves in order to document multifocal demyelination. In the Koski criteria, according to a classification rule, which was derived by a classification and regression tree analysis and applied to 150 patients, the diagnosis of CIDP required that a patient had a chronic nongenetic polyneuropathy, progressive for at least 8 weeks, without a serum paraprotein and either:16
• recordable compound muscle action potentials in ≥75% of motor nerves tested and either an abnormal distal latency in >50% of nerves or abnormal motor conduction velocity in >50% of nerves or abnormal F wave latency in >50% of nerves;
• symmetrical onset of weakness, symmetrical weakness in all four limbs and proximal weakness in ≥one limb.
The Koski criteria have 50–83% sensitivity and 89–97% specificity for typical presentations of CIDP.15–17
Chronic inflammatory demyelinating polyradiculoneuropathy and diabetes mellitus
An association between CIDP and diabetes mellitus (DM) has been reported. Type 2 DM (T2DM) is typically increased in the older population in which CIDP occurs most frequently. However, it is not known whether DM is a major risk for CIDP. Using an epidemiological approach, based on multiple concurrent cases from an Italian population of 4,334,225, the number of expected individuals with CIDP and associated DM was approximated at 13.03, which corresponded to a standardized morbidity ratio (SMR) of 1.07 (95% confidence interval [CI], 0.58–1.80).18 The presence of DM was assessed using the data reported in the clinical records of each patient (clinical history, fasting blood glucose, or reported use of anti-diabetes drugs). In total, 155 patients with CIDP were identified, 14 of whom were also affected by DM (type 1 or 2). An investigation of incidence and prevalence in Olmsted Country (1581 medical records) identified 23 patients with CIDP (19 definite and 4 probable). The incidence of CIDP was 1.6/100,000/year and the prevalence was 8.9/100,000 persons on January 1, 2000. Only one of the 23 CIDP patients (4%) also had DM, whereas 14 of 115 age- and sexmatched controls (12%) had DM.6
The findings of these studies therefore do not support an increased incidence of DM in patients with CIDP.6,18 However, it is possible that some CIDP patients were not identified or that some CIDP cases with associated DM, or preclinical DM, were missed. CIDP may occur with equal frequency in patients with types 1 and 2 DM,19 presenting a diagnostic challenge: both CIDP and diabetes may result in elevated CSF protein.20 Pathologic evaluation clearly differentiates diabetic lumbosacral radiculoplexus neuropathy (DLRPN) from CIDP. These patients would not meet electrophysiologic or clinical criteria for CIDP. This study concluded that the painless motor neuropathy seen in diabetic patients represents painless DLRPN and not CIDP.21 However, a consideration of concomitant CIDP in patients with DM is important since treatment of demyelinating neuropathy would limit disability. It is key to consider a diagnosis of CIDP when diabetic patients develop relatively symmetrical proximal weakness or rapid worsening of neuropathy despite good glycemic control.
In an observational, retrospective study of CIDP patients with (n=67) and without (n=67) DM, those with concomitant DM showed more severe clinical and electrophysiological neuropathy, based on higher lower limb vibration potential thresholds (p = 0.004), higher Toronto Clinical Neuropathy Scores (p = 0.0009), more proximal weakness (p = 0.03), more gait abnormalities (p = 0.03), and more abnormal nerve conduction study findings. Subjects with CIDP and DM also had more abnormal sural nerve conduction studies with lower sural sensory nerve action potential amplitudes (2.4 ± 3.0 μV, 6.6 ± 6.0 μV, p<0.0001) and slower sural nerve conduction velocities (38.6 ± 5.4 m/s, 41.0 ± 5.3 m/s, p = 0.04.22 Patients with DM were less likely to receive specific/disease-modifying therapy although their response rates to CIDP treatment were similar in comparison with those who did not have DM. In particular, the duration of neuropathy rather than the DM status was associated with treatment response. Responders had a shorter CIDP duration than the nonresponders (8.0 ± 6.0 years versus 11.9 ± 7.6 years, p=0.004).
Differential Diagnosis
Neurological examination investigating sensory, motor, and autonomic signs help to define the topography and nature of neuropathy. Principal laboratory studies to support of the diagnosis of CIDP are CSF examination, nerve conduction studies, and nerve biopsy.23 If a paraprotein is detected on serum immunofixation or serum kappa lambda free light chain ratio, a lymphoproliferative disorder, such as osteosclerotic myeloma or lymphoma, should be considered.23
There is no biomarker for CIDP and differential diagnosis can be challenging as hereditary, toxic, metabolic, and neoplastic neuropathies must be considered. In a retrospective study of 59 patients who had been referred with a diagnosis of CIDP, patients were classified into whether or not they had CIDP according to the EFNS/PNS criteria.24 Nearly half (47%) of these patients failed to meet the minimal EFNS/ PNS diagnostic requirements. Another study of patients, diagnosed with CIDP and referred after not responding to initial trials of treatment, found that 54% of patients had an alternative diagnosis, the most common of which was amyotrophic lateral sclerosis. Forty-six percent of refractory patients had CIDP though, and the majority responded to escalating immunotherapy.25 Common diagnostic errors included dependence on subjective treatment benefit, imprecise electrophysiological interpretation of demyelination, and placing too much importance on mild or moderate cytoalbuminologic dissociation. There is also a large group of patients with presumed CIDP, who do not fulfill any of the proposed electrodiagnostic criteria.26 Examples of some confounding diagnoses include:
Guillain–Barré syndrome (GBS) is a monophasic disorder that includes a spectrum of acute autoimmune peripheral neuropathies. It is less common than CIDP and frequently preceded by a triggering event including viral and diarrheal illness. CIDP has a much slower onset than GBS and a more chronic course. The first symptoms in GBS are typically pain, numbness, paresthesia, or weakness in the limbs.27,28 Main features include rapidly progressive bilateral and relatively symmetric weakness of the limbs and reduced or absent tendon reflexes in the affected extremities. Respiratory muscles, cranial nerves, and autonomic nerves are often affected in GBS.28 Patients with CIDP may show a GBS-like onset and a CIDP diagnosis should be considered when a patient thought to have GBS relapses or progresses beyond 8 weeks from onset or when deterioration occurs three times or more.4
Multifocal motor neuropathy (MMN) is an acquired immune-mediated neuropathy that is characterized by chronic or stepwise progressive asymmetrical weakness without sensory deficits. In contrast to CIDP, the motor deficit in MMN tends to be predominantly in the arms, distal more than proximally, and an asymmetric or multifocal nerve involvement. MMN is characterized electrophysiologically by multifocal conduction block across non-entrapment sites with preservation of sensory conduction in the affected nerve segment. At least half the patients have a polyclonal-immunoglobulin (Ig) M anti-ganglioside antibody, and CSF protein is usually normal or slightly elevated. Abnormal MRI T2 signal of the brachial plexus has been reported to occur in 30-40% of patients with MMN who have distal upper extremity weakness.2 Neuropathy ultrasound may be a useful addition as a diagnostic tool.29
Distal, Acquired Demyelinating Symmetric (DADS) neuropathy with myelin-associated-glycoprotein (MAG) antibody: CIDP is typically characterized by proximal and distal weakness, but CIDP variants include a distal phenotype. The variant of DADS with no MAG antibody is a form of atypical CIDP. Differential diagnosis of DADS neuropathy includes neuropathy associated with an IgM monoclonal protein binding to MAG antibody, though some have paraprotein without MAG reactivity. IgM MAG neuropathy is not considered to be an atypical CIDP but another entity, which does not respond to first-line treatment for CIDP.30
Chronic ataxic neuropathy with ophthalmoplegia, M-protein, cold agglutinins and disialosyl antibodies (CANOMAD) is a rare disorder with severe sensory ataxia and cranial nerve involvement.31 This includes ophthalmoplegia, dysphagia, or dysarthria and minimal weakness. CANOMAD typically progresses over years and is associated with IgM antibodies to ganglioside disialosyl moieties, such as GD1b.
Polyneuropathy, Organomegaly, Endocrinology, Monoclonal gammopathy and Skin change (POEMS): in a review of data collected between 2000 and 2010, 60% of patients with POEMS syndrome had originally been diagnosed with CIDP because, in many patients with POEMS syndrome, the other manifestations are subtle, and the primary problem is the demyelinating neuropathy.32 Correct diagnosis was only made after the patients failed to respond to the standard treatment. POEMS syndrome is associated with plasma cell dyscrasia of an IgA or IgG lambda paraprotein and a spectrum of multisystem clinical features.33 It presents with neuropathy typified by motor and sensory
involvement with axonal and demyelinating features. Neuropathic pain may be present32 and pain in the legs is found in 76% of patients with POEMS syndrome compared with only 7% of patients with CIDP.32 POEMS syndrome is typically associated with osteosclerotic myeloma, and, occasionally, with Castleman disease or monoclonal gammopathy of unknown significance.34,35 POEMS syndrome can sometimes be distinguished from CIDP by the clinical profile,32 as well as the presence of a lambda monoclonal protein. On electrodiagnosis, demyelinating changes occur typically in CIDP in a multifocal pattern with more uniform changes in POEMS syndrome.36 Elevated levels of vascular endothelial growth factor may also be helpful in making a diagnosis of POEMS syndrome. A skeletal survey is recommended in patients with a demyelinating polyneuropathy and a lambda monoclonal protein to detect osteosclerotic lesions.37–39
Treatment
Corticosteroids
The EFNS/PNS guidelines recommend that a trial of corticosteroids, IVIG, or plasmapheresis should be considered in all patients with significant disability.12 Supporting evidence in an unblinded, randomized, controlled trial (n=28), showed that prednisone treatment led to a small but significant improvement over no treatment in scored neurological disability, some measures of computer-assisted sensory detection threshold, graded muscle strength, and some attributes of nerve conduction.40,41 Pulsed oral dexamethasone therapy showed equal efficacy to oral prednisolone in a 6-month randomized clinical trial.42 Steroids are accepted as firstline therapy for those patients with sensory and motor dysfunction who can tolerate steroids but should not be used in patients who present with motor variant CIDP, in which corticosteroids are reported to cause worsening.12,43,44 Pure motor CIDP can sometimes be confused with MMN, in which corticosteroid treatment can also result in clinical worsening. The long-term use of corticosteroids is associated with numerous side effects, some serious. These include osteoporosis, skin fragility, weight gain, diabetes, worsening hypertension, hip fractures, cataracts, sleeplessness, and cushingoid appearance.41
Intravenous immunoglobulin
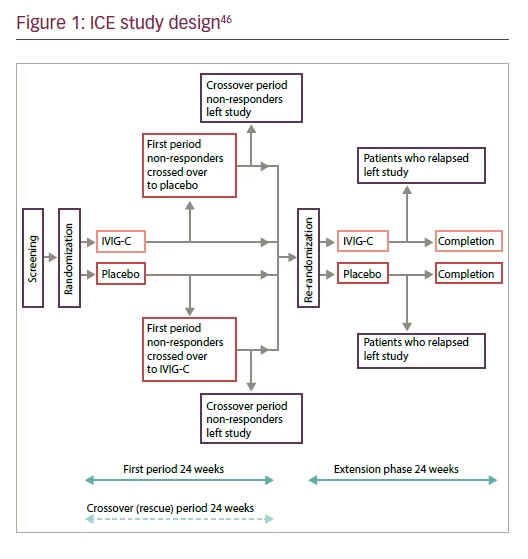
IVIG has been an accepted as first-line therapy for the treatment of CIDP over the last 20 years and is supported by Class I evidence.12,45 The largest trial reported of any CIDP treatment is the ICE trial, which is a randomized, double-blind, placebo-controlled, response-conditional crossover trial of 117 patients with CIDP (Figure 1).46 The aim of the ICE trial was to establish whether immune globulin injection (human), 10%, caprylate/ chromatography purified (IVIG-C; GAMUNEX®-C, Grifols Therapeutics Inc., Clayton, North Carolina, US) has short- and long-term benefit. The trial utilized a baseline loading dose of 2 g/kg over 2–4 days and then a maintenance infusion of 1 g/kg over 1–2 days, every 3 weeks, for up to 24 weeks. Participants who completed the first period or crossover period and whose improved INCAT disability score was consistently ≥1 point greater than at baseline were eligible for inclusion in a 24- week, double-blind extension phase. Eligible participants were randomly re-assigned in a 1:1 ratio to receive 1 g/kg IVIG or placebo over 1–2 days, every 3 weeks, for up to 24 weeks (no loading dose was given), and the adjusted INCAT disability score was assessed every 3 weeks during this period. The primary outcome measure was the INCAT disability score, which is a 10-point ordinal measure that captures changes in daily arm and leg activities and mobility. Secondary outcome measures included grip strength; Medical Research Council (MRC) score, an evaluation of muscle strength; and 36-item short-form survey (SF-36), a quality of life measure.47 During the initial period 54% (32 out of 59) patients treated with IVIG showed improvement in adjusted INCAT disability score, a measure of activities of daily living function, that was maintained to week 24 compared with 21% (12 out of 58) who achieved improvement with placebo (treatment difference: 33.5%, 95% CI 15.4–51.7; p=0.0002). Similar findings were observed during the cross-over period. During the extension phase (6-month follow-up) those who continued to receive IVIG showed decreased relapse compared with those who had received placebo (p=0.011). A significant improvement was seen earlier in grip strength (as early as day 16) compared with changes on the INCAT disability scale in patients who had received IVIG versus those who were given placebo.48
Timing of response to intravenous immunoglobulin
In the ICE trial, 58 patients received placebo and 59 received IVIG administered as a 2 g/kg loading dose over 2–4 days followed by a maintenance dose of 1 g/kg over 1–2 days every 3 weeks, for up to 24 weeks. Among the 30 patients who responded to IVIG, 14 (47%) had an improved adjusted INCAT score by week 3. A further 16 (53%) improved after a second infusion at week 6. Although not considered responders in the ICE trial, an additional two patients improved with IVIG beyond the 6-week window; the latter was an a priori stipulation of response in the ICE trial. In clinical practice, patients may take longer than 6 weeks to respond. The novel responseconditional, crossover study design of the ICE trial required that patients cross over to the alternative treatment if they failed to improve or at the first sign of deterioration or if they were unable to maintain improvement at any time after 6 weeks (Figure 1).49 This design addressed concerns about lack of clinical equipoise, which were raised by the physicians interested in participating in the trial.49 The magnitude of change in CIDP outcome measures required to correlate with a perception of a clinical improvement in the ICE trial has been described through minimum clinically important differences analysis.50
Adverse events related to intravenous immunoglobulin
In the ICE trial, the most common adverse reactions with GAMUNEX®-C (immune globulin injection [human], 10% caprylate/chromatography purified) were headache, fever, chills, hypertension, rash, nausea, and asthenia, and the most serious adverse reaction in clinical studies was pulmonary embolism (PE) in 1 subject with a history of PE. The frequency of adverse events, including serious adverse events, did not seem to depend on age, weight, CIDP severity, or previous IVIG exposure. Although no definitive studies have been carried out exploring mitigation of IVIGrelated side effects, slowing or temporarily discontinuing the infusion and symptomatic therapy with analgesics, nonsteroidal anti-inflammatory drugs, antihistamines, and glucocorticoids may improve some IVIGassociated side effects.51
In the ICE study, IVIG also led to health-related quality of life improvements.52 In the first period, compared with placebo, greater improvements were observed in both SF-36 physical and mental component score (difference: 4.4 points; 95% CI, 0.7–8.0). In addition, participants who received IVIG showed a larger improvement in the Rotterdam Handicap Scale compared with those who received placebo (difference, 3.4 points; 95% CI 1.4–5.5; p=0.001).
Please see the Important Safety Information about GAMUNEX-C on pages 5-6 and refer to the brief summary of full Prescribing Information53 in the Appendix.
Plasma exchange
Plasma exchange (PLEX) has been established as first-line therapy for CIDP in short-term efficacy studies. PLEX should be considered for initial treatment if patients cannot tolerate corticosteroids or IVIG, or have continued to deteriorate following IVIG or corticosteroids.12 The temporal effect is short term, and an indwelling apheresis catheter may be required. Two small double-blind, sham-controlled, randomized clinical trials totaling 47 participants showed that PLEX provides short-term benefit in around two-thirds of patients but that rapid deterioration often ensues when the PLEX is stopped.54–56 The use of PLEX is associated with adverse events related to difficulty with venous access, use of citrate, and haemodynamic changes.57
Immunosuppressive agents
The EFNS/PNS guidelines conclude that more research is needed before evidence-based recommendation on immunosuppressive treatment can be made; however, these treatments, including IV pulse cyclophosphamide,57,58 may be considered when the response to corticosteroids, IVIG, or PLEX is inadequate.12 Although there have been reports on their potential use by case report or open-label studies, randomized, controlled clinical trials to date have not established efficacy of such agents as primary or as add-on agents in the treatment of CIDP.59,60
Risk of relapse
Electrodiagnostic studies have been carried out on participants in the ICE trial who responded to treatment in the first period and were subsequently re-randomized to placebo in the 24-week extension phase. These data indicated that an increase in the total number of DF, specifically conduction block, may signal an increased risk of relapse after discontinuation of therapy whereas the absence of new demyelination may suggest a decreased risk of subsequent relapse.60
Autoantigens in chronic inflammatory demyelinating polyradiculoneuropathy
Recently, autoantibodies against membrane proteins of the peripheral nerve axons or the myelin sheath have been reported in CIDP. For example, a major component of myelin, protein zero (P0) is a target antigen in some patients with CIDP.61 Antibodies to neurofascin and contactin-1, which are concentrated near the nodes of Ranvier, are found in a small population of patients with CIDP.62 In a few patients, IgG4 antibodies to the paranodal proteins contactin and NF155 have been associated with severe, intravenous immunoglobulin (IVIG)-resistant CIDP.63,64 A subset of IVIG-resistant patients with contactin or NF-155 antibodies have responded to rituximab.65,66 This finding supports the premise that improved understanding of antibody responses in CIDP may open new opportunities for future targeted therapeutic interventions.
Conclusions
CIDP is characterized by progressive weakness and impaired sensory function in the arms and legs, and is caused by demyelination of the peripheral nerves. Different diagnostic criteria are available but without a gold standard. CIDP is a progressive, immune-mediated neuropathy that without treatment can lead to significant disability and in a limited number of patients, death. Comorbid diabetic neuropathy in CIDP patients is an important consideration in clinical deterioration despite adequate immune therapy. IVIG, corticosteroids, and plasmapheresis are first-line therapies for the treatment of CIDP. The choice of a specific therapy for an individual is dictated by several factors, including patient comorbidities and the practice environment. The ICE study indicated benefits for IVIG therapy for reducing disability and functional impairment and improving quality of life. An improved understanding of antibody responses and genetic backgrounds in CIDP may offer new opportunities for targeted interventions.
Important Safety Information
GAMUNEX-C (immune globulin injection [human], 10% caprylate/ chromatography purified) is indicated for the treatment of primary humoral immunodeficiency disease (PIDD) in patients 2 years of age and older, idiopathic thrombocytopenic purpura (ITP), and CIDP.
GAMUNEX-C is contraindicated in patients who have had an anaphylactic or severe systemic reaction to the administration of human immune globulin. It is contraindicated in IgA-deficient patients with antibodies against IgA and history of hypersensitivity.
Severe hypersensitivity reactions may occur with IVIG products, including GAMUNEX-C. In case of hypersensitivity, discontinue GAMUNEX-C infusion immediately and institute appropriate treatment.
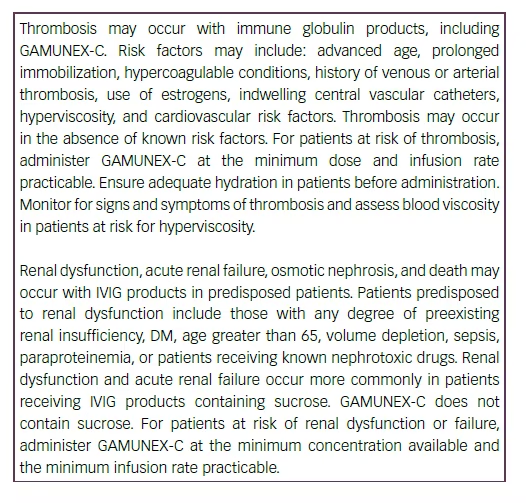
Monitor renal function, including blood urea nitrogen (BUN), serum creatinine, and urine output in patients at risk of developing acute renal failure.
Hyperproteinemia, increased serum viscosity, and hyponatremia may occur in patients receiving IVIG treatment, including GAMUNEX-C.
There have been reports of noncardiogenic pulmonary edema (transfusionrelated acute lung injury [TRALI]), hemolytic anemia, and aseptic meningitis in patients administered with IVIG, including GAMUNEX-C.
The high-dose regimen (1 g/kg x 1-2 days) is not recommended for individuals with expanded fluid volumes or where fluid volume may be a concern.
Because GAMUNEX-C is made from human blood, it may carry a risk of transmitting infectious agents, eg, viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent.
Do not administer GAMUNEX-C subcutaneously in patients with ITP because of the risk of hematoma formation.
Periodic monitoring of renal function and urine output is particularly important in patients judged to be at increased risk of developing acute renal failure. Assess renal function, including measurement of BUN and serum creatinine, before the initial infusion of GAMUNEX-C and at appropriate intervals thereafter.
Consider baseline assessment of blood viscosity in patients at risk for hyperviscosity, including those with cryoglobulins, fasting chylomicronemia/ markedly high triacylglycerols (triglycerides), or monoclonal gammopathies, because of the potentially increased risk of thrombosis.
If signs and/or symptoms of hemolysis are present after an infusion of GAMUNEX-C, perform appropriate laboratory testing for confirmation.
If TRALI is suspected, perform appropriate tests for the presence of antineutrophil antibodies and anti-HLA antibodies in both the product and patient’s serum.
After infusion of IgG, the transitory rise of the various passively transferred antibodies in the patient’s blood may yield positive serological testing results, with the potential for misleading interpretation.
In clinical studies, the most common adverse reactions with GAMUNEX-C were headache, fever, chills, hypertension, rash, nausea, and asthenia (in CIDP); headache, cough, injection-site reaction, nausea, pharyngitis, and urticaria with intravenous use (in PIDD) and infusion-site reactions, headache, influenza, fatigue, arthralgia, and pyrexia with subcutaneous use (in PIDD); and headache, vomiting, fever, nausea, back pain, and rash (in ITP).
The most serious adverse reactions in clinical studies were pulmonary embolism (PE) in 1 subject with a history of PE (in CIDP), an exacerbation of autoimmune pure red cell aplasia in 1 subject (in PIDD), and myocarditis in 1 subject that occurred 50 days post-study drug infusion and was not considered drug related (in ITP).
Please see the brief summary of full Prescribing Information for GAMUNEX-C in the Appendix.







