Vitamin D is an essential nutrient for bone homeostasis that has also been implicated in numerous other disorders, such as cardiovascular disease (CVD) and autoimmune diseases. Originally vitamin D deficiency was associated only with rickets and it was considered that the fortification of food resolved this disorder. However, it is now realised that rickets represents just one manifestation of vitamin D deficiency.1,2 The recommended Dietary Reference Intakes are solely based on the skeletal effects of vitamin D, with the recommended dietary allowance (RDA) ranging from 400 to 800 IU/day depending on age as recommended by the Institute of Medicine to maintain blood levels of 25-hydroxyvitamin D (25[OH]D) of at least 50 nmol/L (20 ng/mL). However to achieve non-skeletal benefits of vitamin D, a multitude of studies have suggested that maintenance of a level of 25[OH]D >30 ng/mL may be required. The majority of primary care clinicians are not aware of the recommended dose for vitamin D supplementation and the optimum level in terms of patients with multiple sclerosis (MS). This article reviews the role of vitamin D in MS and its impact on MS treatment. In addition, the extent and consequences of vitamin D deficiency and guidelines to help the general population and those with MS achieve sufficient levels will be considered.
Vitamin D Deficiency and its Consequences
There is considerable debate on the blood level of 25[OH]D that constitutes deficiency in both the general population and with respect to certain disorders, such as MS. Conflicting recommendations have been published by different organisations. Experts disagree on the optimal 25(OH)D concentration and thus many definitions of deficiency and insufficiency have been proposed. In the general population, a serum level of at least 50 nmol/L 25(OH)D is generally considered to be required for maximum bone health and thus vitamin D deficiency has been defined as a 25(OH)D <50 nmol/L.3,4 The Endocrine Society also recognised that vitamin D had non-skeletal health benefits, and defined vitamin D insufficiency as a 25(OH)D level of 51–74 nmol/L.
Measurement of serum 25(OH)D and not its active form 1,25-dihydroxyvitamin D [1,25(OH)2D] is used to determine vitamin D status. The main reasons are that 25(OH)D has a long half-life of 2–3 weeks compared with 4–6 hours for 1,25(OH)2D, circulates at 1,000 times the concentration of 1,25(OH)2D and in vitamin D deficiency parathyroid hormone (PTH) levels increase the production of 1,25(OH)2D. Thus, patients who are vitamin D deficient often have a normal or even elevated blood level of 1,25(OH)2D. Many assays are available to measure 25(OH)D, but there is a lack of concordance due to considerable intra- and inter-assay variability. The ‘gold standard’ assay is liquid chromatography/tandem mass spectroscopy since it quantitatively measures both 25(OH)D2 and 25(OH)D3. 2, 5
Vitamin D deficiency does not simply occur in the young and elderly but is common across all age groups and is related to a diverse range of disorders, not necessarily bone disorders, such as CVD, autoimmune diseases and schizophrenia. 5 Recent data suggest that vitamin D has a supportive role in the immune system and it has been proposed that it is involved in the prevention of several disease states, including cancer and neurodegenerative disorders.1, 2, 4-7 Attempts to unravel the function of vitamin D have been enhanced by the finding that a proportion of the cells in the body have the vitamin D receptor (VDR) and are able to convert 25(OH)D to the active form 1,25(OH)2D. The VDR is a mediator of 1α,25(OH)2D signalling, and although its regulation is not fully understood, the three main factors that regulate VDR are the environment, genetics and epigenetics. 8, 9 More than 2,000 genes may be directly or indirectly regulated by 1,25(OH)2D.10 In healthy adults who received vitamin D supplementation during the winter, the expression of 291 genes was affected. These genes were responsible for at least 80 different metabolic processes, including alteration of immune function, DNA repair, apoptosis and antioxidant activity. 10 The precise role of these factors should be determined as this may facilitate improved strategies for treating and preventing the diseases associated with VDR function.9
Vitamin D is not toxic even in people taking up to 20,000 IU/d.11 Ekwaru et al. reported that over 17,000 patients were either not taking or taking Vitamin D up to 55,000 IU/day IU/d (with 0.3 % taking over 20,000 IU/d) exhibited no toxicity.12 However, the potential for vitamin D toxicity became apparent in the 1940s when large doses (200,000–300,000 IU/d) were administered to treat rheumatoid arthritis resulting in conditions such as hypercalcaemia, hyperphosphataemia, nephrocalcinosis, kidney stones and soft tissue calcifications. Vitamin D fortification of milk and many foods implemented in the 1920s was banned in most countries in the 1950s due to a perceived association with certain infant abnormalities in the UK, which almost certainly had a different cause (such as sarcoidosis or 24-hydroxylase deficiency).12
The possible toxicity of high doses of vitamin D has been investigated in a number of studies. Dudenkov et al. conducted a retrospective, population-based study (between 2002 and the end of 2011) using the Rochester Epidemiology Project involving those with measured 25(OH)D values >125 nmol/L. If hypercalcaemia (the primary vitamin D toxicity) was recorded within 3 months of 25(OH)D measurement it was considered potentially associated with the 25(OH)D level. A 25(OH) D value of >125 nmol/L was found in 9.2 % of the 20,308 cohort (8.4 %, 0.6 %, 0.2 % for >125, >200 and 250 nmol/L, respectively). There was no significant relationship between serum 25(OH)D level and serum calcium or risk of hypercalcaemia. However, only four cases were identified by medical review where a level of 25(OH)D >125 nmol/L was temporally associated with hypercalcaemia. Only one case had clinical toxicity and was associated with a blood level of 25(OH)D of 910 nmol/l. Thus, although the incidence of 25(OH)D levels >125 nmol/L significantly went up during this period, no corresponding increase in acute clinical toxicity was evident.13 This is consistent with the Endocrine Society’s practice guidelines stating that vitamin D intoxication does not usually occur until levels of >375 nmol/L serum 25(OH)D.4
Vitamin D deficiency and insufficiency (i.e. 25(OH)D <50 and 52.5– 72.5 nmol/L) are now a global problem.4 According to the Institute of Medicine, deficiency was associated with increased risk of mortality. A study of over 13,000 individuals showed that after adjusting for baseline factors, those with levels of <44.5 nmol/L (the lowest quartile) were associated with a 26 % increase in all-cause mortality compared with those with >75.3 nmol/L.14 A recent meta-analysis showed that serum 25(OH)D levels ≤75 nmol/L were associated with a higher allcause mortality than concentrations >75 nmol/L (p<0.01) and this benefit persisted with blood levels of 25(OH)D up to at least 175 nmol/L.15
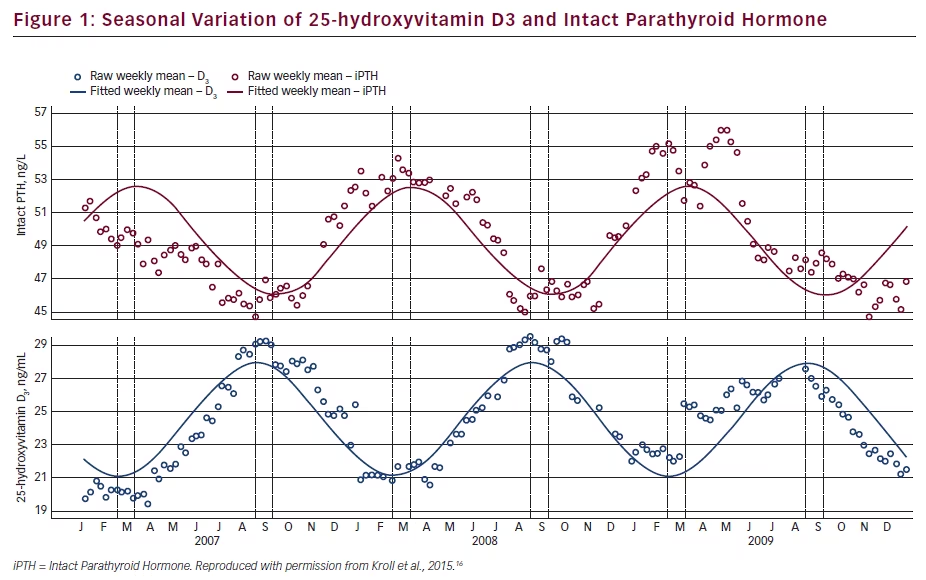
Elevated PTH and vitamin D deficiency are thought to have an impact on bone fragility and other organ systems. A temporal inverse relationship occurs, with PTH being highest in winter when serum 25(OH)D is at its lowest value. Since most of the studies of PTH and vitamin D were of short duration, Kroll et al. investigated their seasonal variation over several years. Weekly mean concentrations were retrospectively determined using 3.8 million results from adults. For all genders and latitudes, a seasonal variation was seen, with peaks throughout September and troughs throughout March. There was an inverted pattern of peaks and troughs for the interpretation of PTH relative to 25(OH)D3 with a 4-week delay (see Figure 1). Both vitamin D deficiency and insufficiency were common (33 % of patients <50 nmol/L; 60 % <75 nmol/mL, as was elevated PHT [33 % >65 pg/mL]). Patients deficient in 25(OH)D seasonally varied between 21 % to 48 % while those with elevated PTH reciprocally varied from 28 % to 38 %. Both 25(OH)D and PTH levels vary in a sinusoidal pattern over the year even in people supplemented with vitamin D2, with 25(OH)D3 higher in the summer and lower in the winter and the reverse for PTH. In addition, most had vitamin D deficiency and secondary hyperparathyroidism. These findings were true for three latitude regions, both genders, multiple years and the presence or absence of detectable 25(OH)D.16
Routine Testing of Vitamin Status
In the US, testing rates for vitamin D status [25(OH)D] seem to be increasing despite the uncertainty on the definition of deficiency. Two studies evaluated the rate of testing and one found that it increased threefold between 2008 and 2010, while the other observed a 50 % increase between 2008 and 2009.17 Primary care clinicians may include blood tests to measure 25(OH)D concentration as part of routine laboratory work and recommend supplementation for the possible prevention of cancer, CVD, diabetes, autoimmune disorders, cognitive decline and other conditions. However, the majority of primary care clinicians are not aware of the recommended dose for vitamin D supplementation and optimum serum levels of 25(OH)D with respect to patients with MS.
The US Preventative Services Task Force conducted a systemic review of the pertinent literature and concluded that data were insufficient to recommend vitamin D screening in routine clinical practice or to assess the effectiveness and overall balance of benefits and risks of supplemental vitamin D for primary prevention of cancer or CVD. This recommendation confirmed the conclusions of the 2010 Institute of Medicine and the
Endocrine Practice Guidelines 2011 on vitamin D supplementation and on prevention and treatment of vitamin D deficiency respectively.3,4,17,18 Manson emphasised that the optimum serum concentration of 25[OH] D is disputed and the putative benefits of vitamin D may be confounded by outdoor physical activity, adiposity and overall nutritional status. However, it is important to target assessment of vitamin D in patients with risk factors for and disorders associated with deficiency, such as osteoporosis, and consider treatment. A clearer picture should emerge once the results of several ongoing randomised trials in the US, Australia, Finland and the UK are reported.
The Canadian Agency for Drugs & Technologies in Health evaluated the clinical and cost effectiveness of vitamin D testing in the general population. Testing has increased in developed countries due to the potential link between vitamin D deficiency and adverse health outcomes, but this has not resulted in improved healthcare. Evidencebased guidelines were developed for testing and recommended that it should only be indicated for specific high-risk conditions, such as osteoporosis, rickets, osteomalacia, malabsorption syndromes, renal disease and in those taking medications that may affect vitamin D status.2,4 The authors of the Canadian review concluded that limited evidence of varying quality existed suggesting that vitamin D testing is not warranted in the general population with the majority of studies not identifying direct evidence on the clinical utility of testing.
Interventions for Vitamin D Deficiency and Guidelines for its Management
The RDA for vitamin D is 600 IU/d for individuals aged 1–70 and 800 IU/d for those >70 years, however, this takes no account of body mass index (BMI) or absolute body weight. Body weight is reflected in the Clinical Practice Guidelines by the Endocrine Society with the recommendation that obese individuals receive two to three times more vitamin D although studies to conclusively justify this have been lacking. To address this situation, a study to assess the effect of vitamin D supplementation and body weight in 17,614 healthy adults has been completed. Volunteers were from a preventative health programme that encourages and monitors vitamin D supplementation and determines both 25(OH) D and calcium levels in serum. Vitamin D supplementation ranged from 0–55,000 IU/d with serum 25(OH)D levels from 10.1–394 nmol/L. BMI was a better determinant of 25(OH)D than absolute body weight. In those obese and overweight, average serum 25(OH)D levels were 19.8 nmol/L and 8.0 nmol/L lower compared with normal weight subjects. The study authors recommended two to three times higher vitamin D supplementation for obese people and 1.5 times higher for those overweight relative to normal weight subjects in order to achieve recommended 25(OH)D targets.11
A number of strategies to counter vitamin D deficiency have been published. In the absence of adequate sun exposure, supplementation with either vitamin D2 or D3 is necessary.1,4 Both vitamin D2 and vitamin D3 can be equally effective in maintaining serum 25(OH)D levels.4 Since human milk has a very low vitamin D content (approximately 20 IU/L), supplementation for breast-feeding mothers with 4,000–6,000 IUs daily to satisfy the infant’s requirement and/or infants receiving 400 IUs of vitamin D daily is important.4
Guidelines have been published for the evaluation, treatment and prevention of vitamin D deficiency to assist healthcare professionals in its management. For example, the 2011 Endocrine Society Clinical Practice Guideline4 was particularly targeted towards the care of patients at risk of deficiency. Supplementation was recommended (with vitamin D2 or D3) for those deficient, with daily doses specific to age and clinical circumstances. Screening was only recommended for those at risk of deficiency using determination of 25(OH)D in serum by a reliable
assay as the initial diagnostic test. At the time of the published report, insufficient evidence was available to recommend screening the general population (not at risk of deficiency) or to treat with vitamin D in order to attain the noncalcaemic benefit for cardiovascular protection.
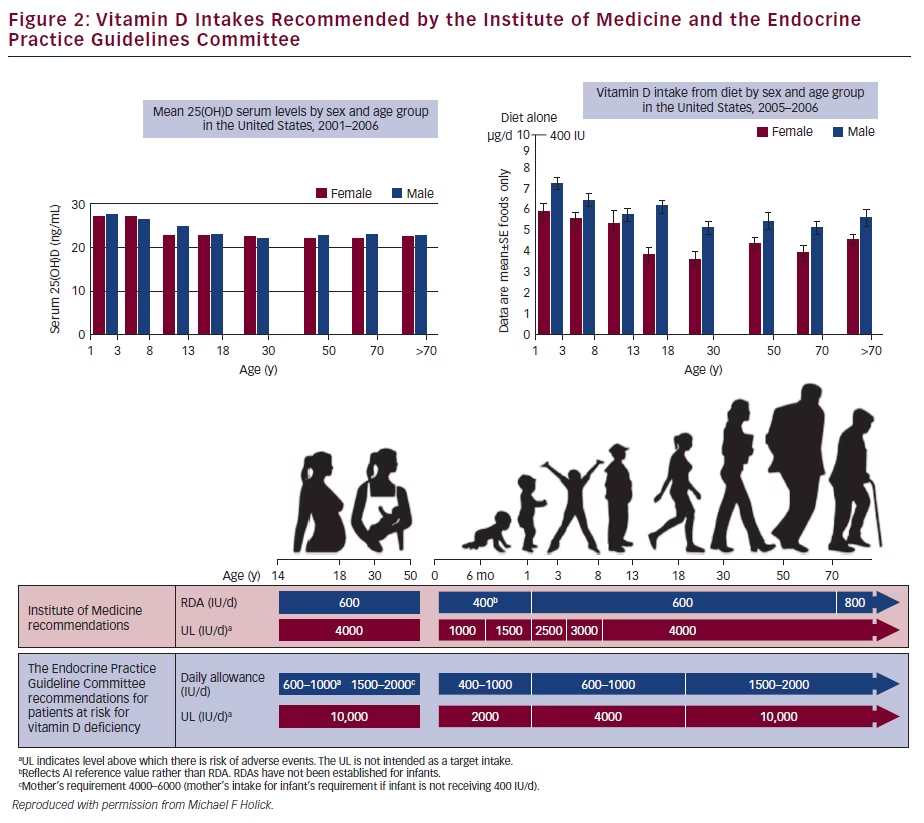
In addition to the controversy surrounding both the definition of vitamin D deficiency and its requirement, the periods when adequate vitamin D is essential during foetal maturation are also not defined.5 This lack of knowledge is at least partly due to the paucity of long-term clinical trials. Figure 2 summarises the recommended dietary allowances of the Institure of Medicine and the Endocrine Society guidelines. As shown in the figure, the dietary intake of vitamin D by age and gender is not sufficient to meet the daily intake recommendations provided by the existing guidelines.
Most of the daily requirement for vitamin D can be obtained from sun exposure although this may not be sufficient to maintain adequate levels. Sun exposure should produce adequate vitamin D3 that is stored in body fat for release during periods of limited sunlight. Vitamin D is present naturally in few foods and in the US children and adults cannot obtain sufficient vitamin D from their diet.19
Since sun exposure and food intake could be insufficient to maintain sufficient levels of vitamin D, management of vitamin D deficiency in children and adults may be needed and can be effectively achieved by administering 50,000 IU vitamin D2 or vitamin D3 once a week for 6 or 8 weeks respectively. Moreover, a dose of 600–1,000 IU/d is effective to prevent recurrence in children and 50,000 IU vitamin D2 or vitamin D3 every 2 weeks (equivalent to approximately 3,600 IU daily) in adults. This strategy maintains blood levels of 25(OH)D at approximately 100– 150 nmol/L for up to 6 years with no evidence of toxicity.4, 20 In pregnant women supplementation with 2,000 and 4,000 IU/d during pregnancy improve maternal/neonatal vitamin D status.5
Vitamin D Effect on the Experimental Autoimmune Encephalomyelitis Model
The positive effect of 1,25-Dihydroxyvitamin D3 (1,25[OH]2D3) in the experimental autoimmune encephalomyelitis (EAE) animal model has been attributed to an anti-inflammatory mechanism, but it could also directly act on neural cells to promote central nervous system (CNS) recovery. Subsequently, it has been shown that neural stem cells (NSC) constitutively express the VDR which can be up-regulated by 1,25(OH)2D3. The proliferation and differentiation of NSCs into neurons
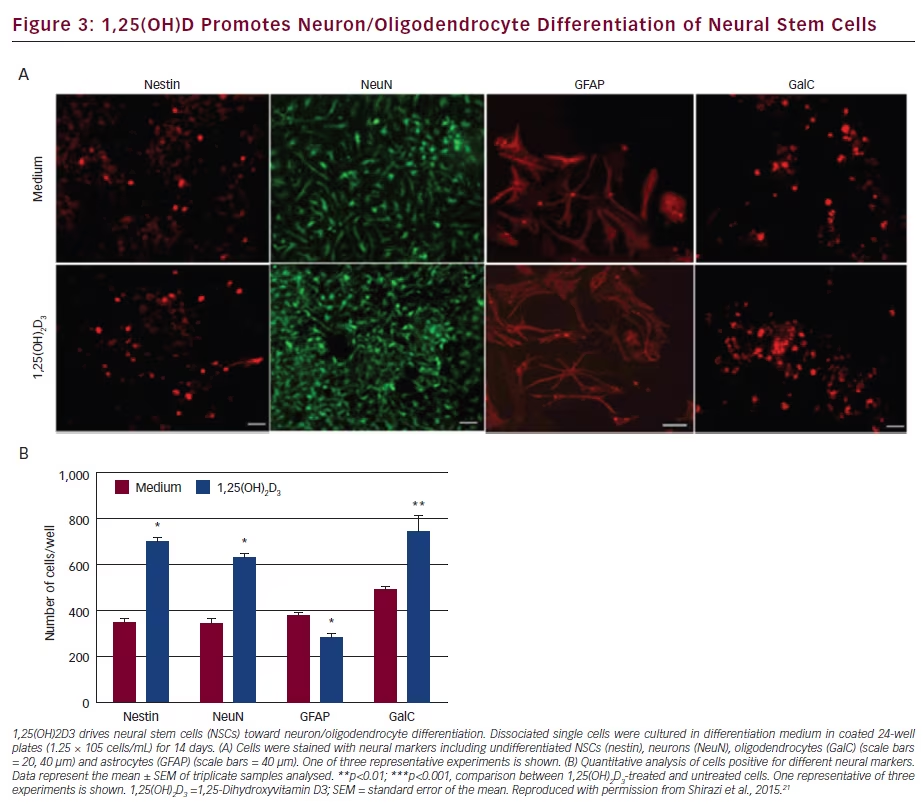
and oligodendrocytes is significantly enhanced by 1,25(OH)2D3 (see Figure 3). Expression of neurotrophic factors (NT-3, BDNF, GDNF, CNTF) needed for neural cell survival and differentiation were increased in NSCs treated with 1,25(OH)2D3. These findings suggest a remyelinating and neuroprotective effect of 1,25(OH)2D3 in EAE and MS.21
Immune Regulation of Vitamin D and Multiple Sclerosis
Ramagopalan et al. investigated responsive regulatory elements in the major histocompatibility complex (MHC) class II region to establish whether vitamin D could interact with inherited factors. A single MHC vitamin D response element (VDRE) localised in the promoter region of major histocompatibility complex, class II, DR beta 1 (HLA-DRB1) was identified. A specific increase in the expression of HLA-DRB1 was observed with vitamin D, but only in HLA-DRB*15-bearing lymphoblastoid cells. These results demonstrate a direct functional interaction between vitamin D and the major locus determining genetic susceptibility and thus provide additional insight into the role of vitamin D as an environmental candidate in MS.22
Considerable evidence indicates that vitamin D has a protective effect on MS, as the risk is reduced with sun exposure, vitamin D supplementation and high serum levels.23 A study of Hispanic patients – 132 with clinically definite MS (CDMS), 58 relapsing-remitting MS (RRMS) in remission, 34 RRMS during a relapse and 40 primary progressive (PP) MS patients plus 60 healthy matched controls – examined the putative regulatory mechanisms of vitamin D in MS pathology.24 Both 25(OH)D and 1,25(OH)2D3 levels were significantly lower in RRMS patients than controls and in those with a relapse compared with during remission. Interestingly, values were similar between PPMS and control patients. In addition, activated vitamin D enhanced the development of interleukin (IL)-10-producing cells and reduced those secreting IL-6 and IL-17.24 Furthermore, 1,25(OH)2D induced VDR expression in activated and resting cells. The expression and biological activity of indoeamine-2,3-dioxygenase was also increased by 1,25(OH)2D, producing a significant increase in CD4+CD25+ T regulatory cells. Thus, vitamin D appears to be important in T cell homeostasis during MS, and supplements to treat the deficiency could be appropriate.24
Vitamin D and the Risk of Multiple Sclerosis
A multicentre, incident, case-control study examined the role of sun exposure and vitamin D status as risk factors for CNS demyelination (first demyelinating events).25 The results indicated that higher levels of exposure to the sun were associated with a lower risk of first demyelinating event especially with higher serum vitamin D status. This finding should be evaluated in clinical trials for MS prevention.
In two large female US cohorts (Nurses’ Health study and Nurses’ Health Study II – 92,253 and 95,310), dietary intake of vitamin D and risk of MS was examined in order to determine if it has a protective effect on MS risk. Follow up was for 20 or 10 years. Assessment of diet was made at baseline and every 4 years. In total, 173 cases of MS occurred during follow up. Patients in the highest quintile of total vitamin D intake at baseline had a lower risk for MS (pooled age-adjusted relative risk [RR] for MS of 0.67) versus those in the lowest quintile. There was an inverse association with MS risk in those obtaining vitamin D from supplements (≥400 IU/day) compared with women not taking supplements (RR 0.59). There was no effect on incidence of MS and vitamin D from food. These findings suggest a protective effect of vitamin D intake on risk of developing MS.26
Vitamin D and the Clinical Course of Multiple Sclerosis
A number of studies have investigated the possible link between vitamin D and the risk of developing any type of MS, conversion from clinically isolated syndrome (CIS) to CDMS and on the course of CDMS have all been assessed.
Although higher 25(OH)D levels have shown a protective association with MS onset, there are few studies investigating whether it can affect the clinical course. In a prospective, population-based study of 145 RRMS patients mostly treated with immunosuppressive therapy, an inverse relationship between 25(OH)D level and hazard of relapse over the subsequent 6 months was observed for each 10 nmol/L increase in 25(OH)D (hazard ratio [HR] 0.91, 95 % confidence interval [CI] 0.85–0.97), giving a 12 % reduction in risk of relapse. Thus clinically raising 25(OH)D levels by 50 nmol/L could halve the hazard of a relapse.27
The effect of vitamin D on exacerbation risk in MS was examined in another prospective longitudinal trial that recruited 73 RRMS patients.28 Serum 25(OH)D levels were assessed every 8 weeks. In total, 139 exacerbations occurred over a mean follow-up of 1.7 years. Levels of 25(OH)D were categorised into low (<50 nmol/L), medium (50–100 nmol/L) and high (>100 nmol/L). A significant decrease in exacerbation risk with higher vitamin D levels was observed with relative exacerbation rates 0.7 and 0.5 (p=0.007 for trend), respectively, for medium and high levels compared with low levels. There was a 27 % decrease in the exacerbation rate for each doubling of serum 25[OH]D concentration suggesting a beneficial effect of vitamin D on MS disease course in RRMS patients. The study investigators emphasised the possibility of reverse causality and suggested that randomised intervention studies were needed to confirm the effect of supplementation of vitamin D in MS patients.28
The role of vitamin in conversion from CIS to MS has also been studied. The Multiple Sclerosis Genetics – Expression, Proteomics, Imaging, Clinical (EPIC) study examined the relationship between vitamin D status and the development of new T2 or contrast-enhancing lesions on brain MRI in MS patients.29 In this 5-year longitudinal cohort study of patients with CIS or RRMS, multivariate analysis showed that each 20 nmol/L increase in 25(OH)D was associated with a 15 % lower risk of new T2 lesions and a 32 % lower risk of gadolinium-(Gd)-enhancing lesions. In addition, each 20 nmol/L higher vitamin D level was associated with a lower relapse rate. Thus the 25(OH)D levels are inversely associated with MS activity on brain MRI supporting the need for a randomised trial of vitamin D supplementation.29
In the Betaferon/Betaseron in Newly Emerging multiple sclerosis For Initial Treatment (BENEFIT) study 465 patients with CIS were tested for 25(OH)D levels over 2 years and followed for 5 years to determine whether change in vitamin D level or status predicted disease activity and prognosis.29, 30 The average serum 25(OH)D level in the first 12 months strongly predicted MS activity and progression in the next 4 years. The probability of CDMS was lower in all patients with levels of 25(OH)D ≥50 nmol/L and in the subgroup of patients starting with IFNb-1b compared with those with levels <50 nmol/L (p=0.048 and p=0.0043 after 1 year for all patients and IFNb-1b treated patients). MRI activity (cumulative number of new active lesions) was significantly reduced in all patients and in the subgroup treated with IFNb-1b with ≥50 nmol/L 25(OH)D levels versus those with levels <50 nmol/L (p=0.002 and p=0.0055, respectively). Furthermore, there was a strong inverse association between the percent change in T2 volume and brain volume and 25(OH)D levels in all patients (p=0.008 and 0.005, respectively) and the subgroup of patients starting with early treatment with IFNb-1b (p= 0.016 and 0.0037, respectively).30,31 These results indicate that a low 25(OH)D serum concentration early in MS in patients mainly treated with IFNb-1b is a risk factor for progression over the long term.30
These findings emphasise the importance of identifying and correcting 25(OH)D insufficiency early in the course of MS and also suggest that early IFNβ-1b treatment has an additive effect with 25(OH)D in reducing disease severity and progression in both clinical and imaging outcomes without impacting tolerability to IFNb-1b.
An Australian study of 178 CDMS patients treated with IFNb examined if there is an association between treatment and the level of vitamin D and if there is an interaction and an effect on relapse rate.32 A significantly higher mean 25(OH)D level was seen in those treated with IFNb than untreated patients (p<0.001) due to treated patients realising approximately a threefold increase in 25(OH)D per hour of sun exposure compared with those untreated. Furthermore, an association between 25(OH)D and reduction in relapse rate was only seen in treated patients (p<0.001). This positive effect on relapse rate was only evident in patients with higher 25(OH)D levels (HR 0.58, 95% CI 0.35–0.98). By contrast, the relapse rate increased in treated patients insufficient for 25(OH)D. These findings indicate that one of the explanations for the effects of IFNB on relapse rate may be due to of vitamin D metabolism. Thus monitoring vitamin D status and supplementation should be considered in patients on IFNb therapy.32
The potential beneficial effect of supplementing IFNb-treated MS patients with vitamin D has been demonstrated in a 1-year study of 66 MS patients. In the vitamin D group, 25(OH)D in serum increased to a mean of 110 nmol/L from 54 nmol/L, and 84 % achieved a level >85 nmol/L versus 3 % in placebo patients (p<0.0001). There was a reduction in new T2 lesions (p=0.286), disability accumulation (p=0.071) and significantly lower T1-enhancing lesions (p=0.004) with vitamin D supplementation, but no significant difference in adverse events (AEs) or annual relapse rate.33
In order to evaluate the effect on vitamin D before and during treatment with IFNb, 88 RRMS patients were regularly tested for 25(OH)D levels and had MRI scans in the 6 months before and during therapy.34 Each 10 nmol/L increase in 25(OH)D reduced the odds for new T1 Gd-enhancing lesions, new T2 lesions and combined unique activity by 12.7 % (p=0.037), 11.7 % (p=0.044) and 14.1 % (p=0.024). Of note, an association between 25[OH]D levels and disease activity was not observed after initiation of treatment, and HLA-DRB1*15 status did not affect the outcome. 34
The CLIMB study assessed whether higher levels of 25(OH)D were protective in patients treated with glatiramer acetate since this has been shown with IFNb.35 In total, 247 RRMS patients receiving either GA or IFNb as their first disease-modifying drug (DMD) were tested for 25(OH)D. Higher 25(OH)D significantly predicted longer time to first event (clinical and MRI endpoint) in the IFN group (p=0.013) but not with GA (P=0.38). However, this study has a number of limitations, including a small sample size and its observational design, so causality may not be implied or addressed. There are no data available from randomised head-to-head clinical trials documenting differences in MRI parameters based on vitamin D levels with these drugs.
In a large international multicentre study, clinical and biochemical variables that may predict conversion from CIS to CDMS were explored in 1,047 CIS patients over at least a 2-year follow up.36 The strongest independent predictors of conversion to CDMS were MRI lesion load, oligoclonal bands and age at CIS. Although lower 25(OH)D levels in univariable analysis were associated with CDMS, this was attenuated in the multivariable model. It can be concluded that based on the interpretation of the data, the possible role of vitamin D required further investigation.36
Possible Mode of Action for the Effect of Vitamin D and Interferon in the Treatment of Multiple Sclerosis Patients
The possible mechanistic rationale for the potential clinical effects of 25(OH)D in MS was studied over 2 years in CIS patients initiated on IFNb-1b.37 Serum 25(OH)D and global gene expression were evaluated and MS disease activity was assessed by the number of Gd-enhancing lesions on MRI. A significant inverse association between 25(OH)D levels and number of Gd-enhancing lesions was observed. Increased 25(OH)D levels were associated with a decrease in MS activity. A number of known gene targets of IFNb-1b and a regulator of sphingosine-1-phosphate bioavailability were identified in the complex network of genes regulated by 25(OH)D. Two gene sets were identified: one for regulation by 25(OH)D and another associated with IFNb-1b treatment. In addition, the 25(OH)D effects on MS activity were additively enhanced by IFNβ-1b. Single genes associated with 25(OH)D levels reduced the gadolinium-enhancing lesion count more effectively in combination with IFNβ-1b, which paralleled the additive clinical effect. These results indicate the potential benefit of monitoring and supplementing vitamin D in early MS patients treated with IFNb-1b.37
The expression of immunoglobulin-like transcript (ILT) 3 and 4, which are receptors that inhibit immune responses, is affected by IFN. This may be a possible mechanism by which IFN exerts its therapeutic effect in MS. Waschbisch and colleagues investigated this effect by measuring the expression of ILT3 and 4 on immune cells from both MS patients and post-mortem brain tissue.38 In addition, the ability of IFN or in combination with vitamin D to induce up-regulation of these receptors in vitro was studied and the expression levels from IFNtreated and untreated MS patients compared. The results indicated that in vitro IFNb up-regulated ILT3 and ILT4 on monocytes while 1α,25 dihydroxyvitamin D3 (1α,25[OH]2D3) increased expression of ILT3, but not ILT4. Moreover, in demyelinating lesions in post-mortem MS brains, ILT3 was abundant, and expression on monocytes in the cerebral spinal fluid was higher than in peripheral blood. These findings suggest that either ILT3 is induced by the CNS milieu or ILT3-positive monocytes preferentially enter the brain and both ILT3 and ILT4 may have a pivotal role in immune responsiveness (regulation of neuroinflammation) in MS by IFN and vitamin D.38
Conclusion
There is a growing realisation that vitamin D has a pivotal role in numerous processes in the body in addition to its effects on bone health being involved in the prevention of a number of acute and chronic disorders. However, the majority of primary care clinicians are not aware of the recommended dose for vitamin D supplementation and optimum serum level with respect to patients with MS. Considerable evidence suggests that vitamin D deficiency is common in the general population globally. Several vitamin D supplementation strategies have been developed to counter this problem. It is also known that high levels used as supplements have not shown acute clinical toxicity, so adverse events are likely to be very rare in the general population. However, vitamin D supplementation is hampered by the lack of agreement on the optimum serum concentration of 25(OH)D.
The amount of sun exposure and level of vitamin D are risk factors for CNS demyelination. 1,25(OH)D enhance the differentiation of NSCs into neurons and oligodendrocytes and has a role in neural cell survival, which may indicate a neuroprotective effect in EAE and MS. A number of studies have revealed a beneficial relationship between higher vitamin D status and lower risk of MS and an inverse association with brain MRI. However, whether high 25(OH)D levels have a beneficial effect on the MS disease course is yet to be conclusively proved, although evidence is emerging to support this hypothesis. Data have been published indicating that lower 25(OH)D levels are associated with CIS conversion to CDMS.
Gene expression studies have indicated that increased 25(OH)D levels regulate expression of the large gene–gene interaction system involved in the regulation of immune-modulating processes for MS activity. Immunoinhibitory ILT3 and 4 on monocytes are up-regulated by IFNb and 1,25(OH)2D3 increased expression of ILT3, but not ILT4, suggesting that both IFNβ and 1,25(OH)2D3 play a role in modulating the immune system in MS. In addition, vitamin D3 reduces MRI disease activity when added to IFNβ-1b therapy, and this may be a useful add-on treatment strategy in MS. So far, this beneficial effect has not been observed with other DMDs, such as GA.
In conclusion, although a number of specific questions concerning supplementation and serum levels of 25(OH)D have not yet been conclusively addressed, growing mode of action and clinical evidence appears to support the importance of vitamin D levels in the clinical and MRI course of MS. Furthermore, evidence seems to support the positive additive effect of vitamin D in the course of MS both alone and in patients taking IFN.